Product Description
This is the most comprehensive ISO 13485 toolkit currently available.
The ISO 13485 Toolkit is designed in Microsoft Office format and can be customized to meet your organization’s unique requirements. Along with a standard format and content, the ISO 13485 template documents feature sample text that is distinctly marked to show the kind of information that your organization needs to provide. Complete example documents are also included to assist you with implementation.
Authored by an experienced auditor with over two decades in Quality Management, our toolkit distills a wealth of expertise into a user-friendly format.
With quality and quantity included, this award-winning toolkit covers everything an organization will need, so you can use it first to become certified to the standard, and then to develop and continually improve your Medical Devices Quality Management System.
What is included within the ISO 13485 toolkit?
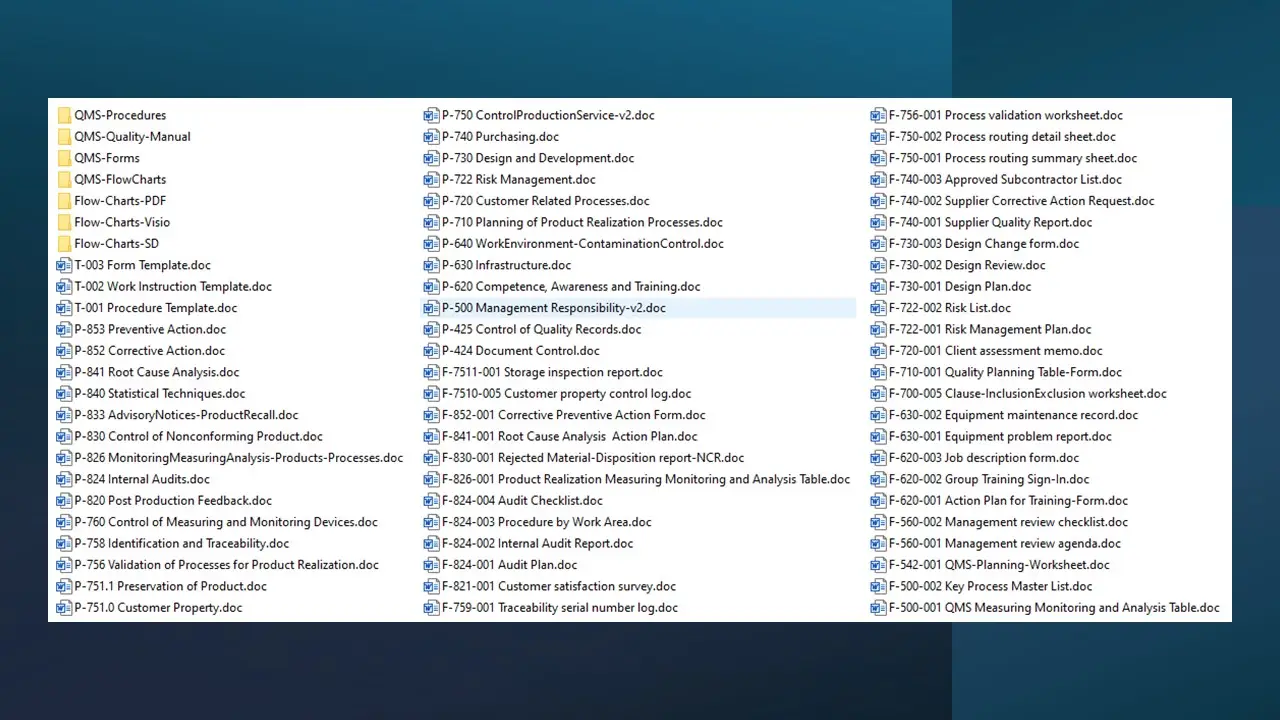
- Over 140 template documents, encompassing policies, procedures, controls, checklists, tools, and various other valuable documentation.
- Available as an instant download after purchase
140+ Templates
Medical Devices Quality Management System Documentation pack
A complete and thorough set of documentation designed to assist clients, consultants, and service providers in successfully achieving ISO 9001:2015 certification.
Pack folder structure:
- QMS-FlowCharts
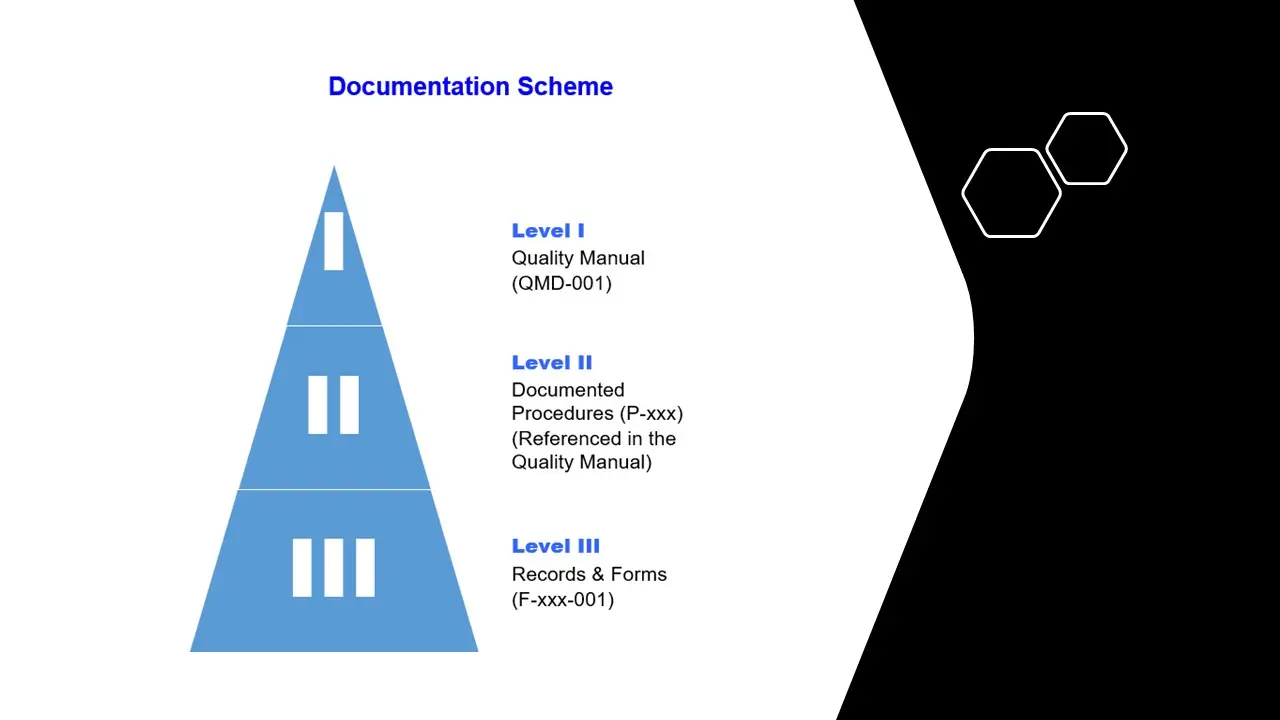
- QMS-Forms
- QMS-Procedures
- QMS-Quality-Manual
List of all documents:
- Quality Policy.Doc
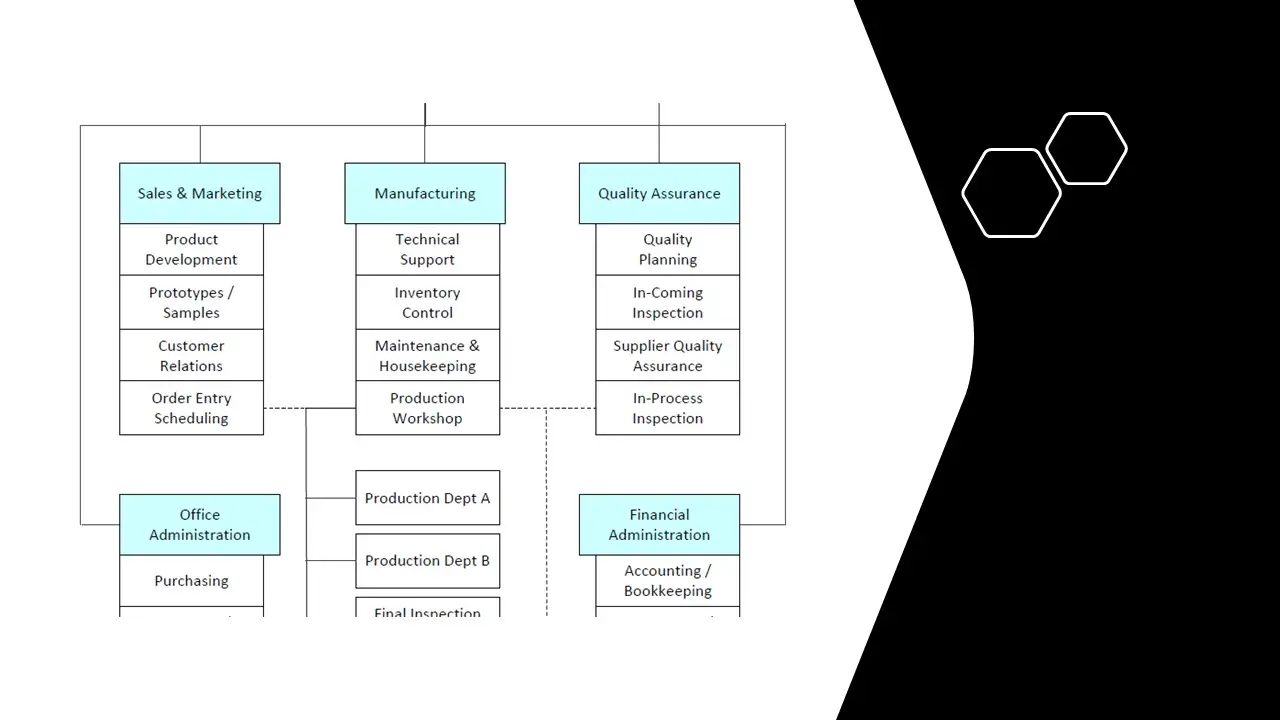
- Organization Chart.Doc
- Flow Chart For Iso Qm.Pdf
- Software Inventory.Doc
- Document Change Request.Doc
- Document Revision Checklist.Doc
- Quality Records Table-V2.Doc
- Qms Measuring Monitoring And Analysis Table.Doc
- Key Process Master List.Doc
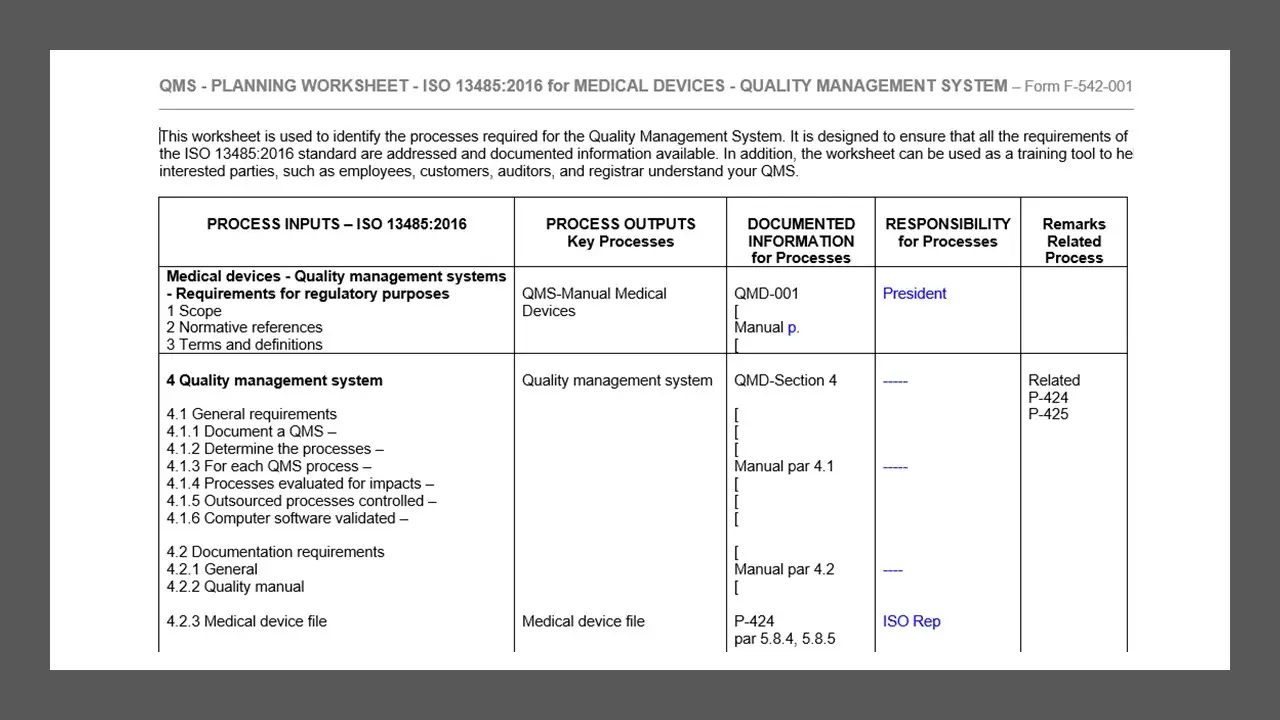
- Qms-Planning-Worksheet.Doc
- Management Review Agenda.Doc
- Management Review Checklist.Doc
- Action Plan For Training-Form.Doc
- Group Training Sign-In.Doc
- Job Description Form.Doc
- Equipment Problem Report.Doc
- Equipment Maintenance Record.Doc
- Clause-Inclusionexclusion Worksheet.Doc
- Quality Planning Table-Form.Doc
- Client Assessment Memo.Doc
- Risk Management Plan.Doc
- Risk List.Doc
- Design Plan.Doc
- Design Review.Doc
- Design Change Form.Doc
- Supplier Quality Report.Doc
- Supplier Corrective Action Request.Doc
- Approved Subcontractor List.Doc
- Process Routing Summary Sheet.Doc
- Process Routing Detail Sheet.Doc
- Customer Property Control Log.Doc
- Storage Inspection Report.Doc
- Process Validation Worksheet.Doc
- Traceability Serial Number Log.Doc
- Equipment List.Xls
- Customer Satisfaction Survey.Doc
- Audit Plan.Doc
- Internal Audit Report.Doc
- Procedure By Work Area.Doc
- Audit Checklist.Doc
- Product Realization Measuring Monitoring And Analysis Table.Doc
- Rejected Material-Disposition Report-Ncr.Doc
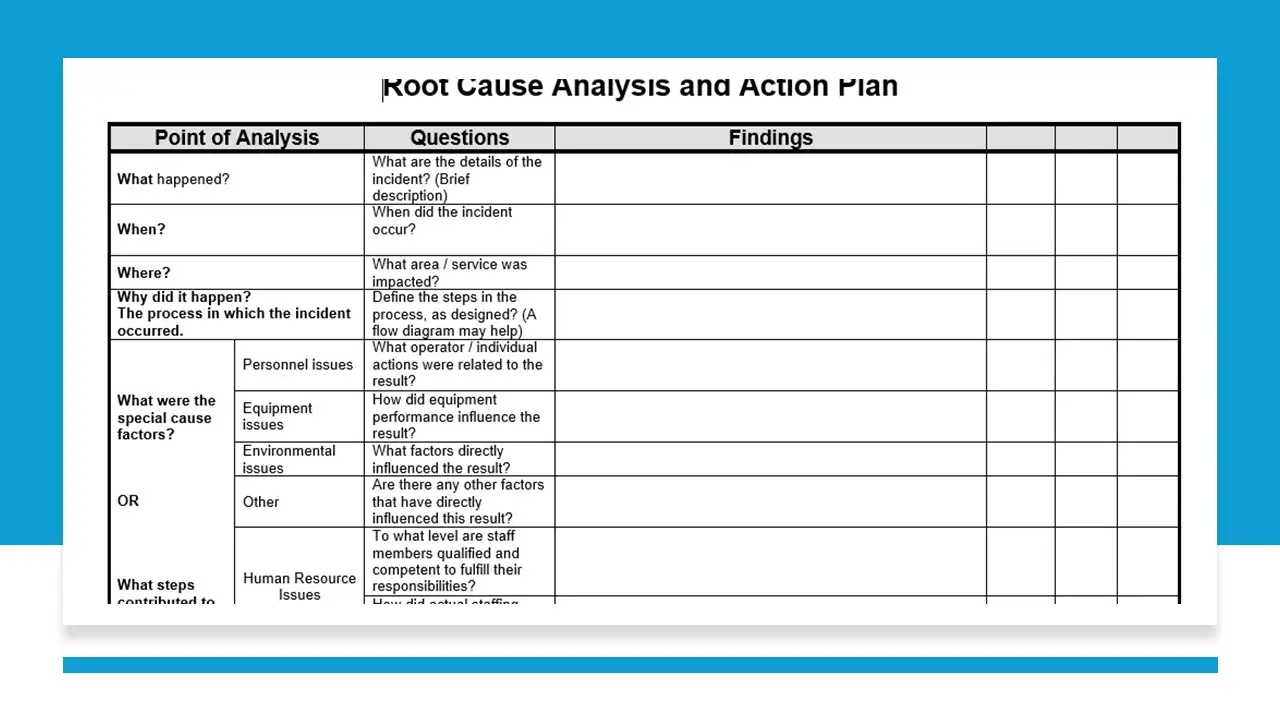
- Root Cause Analysis Action Plan.Doc
- Corrective Preventive Action Form.Doc
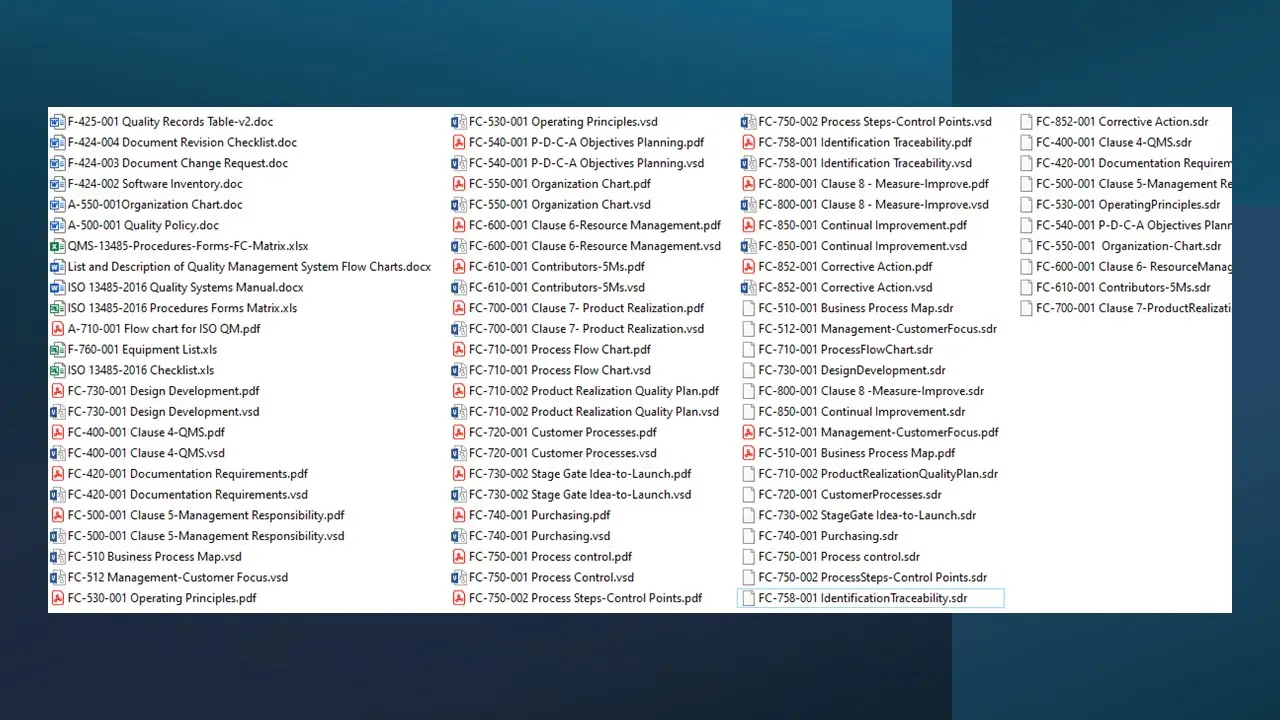
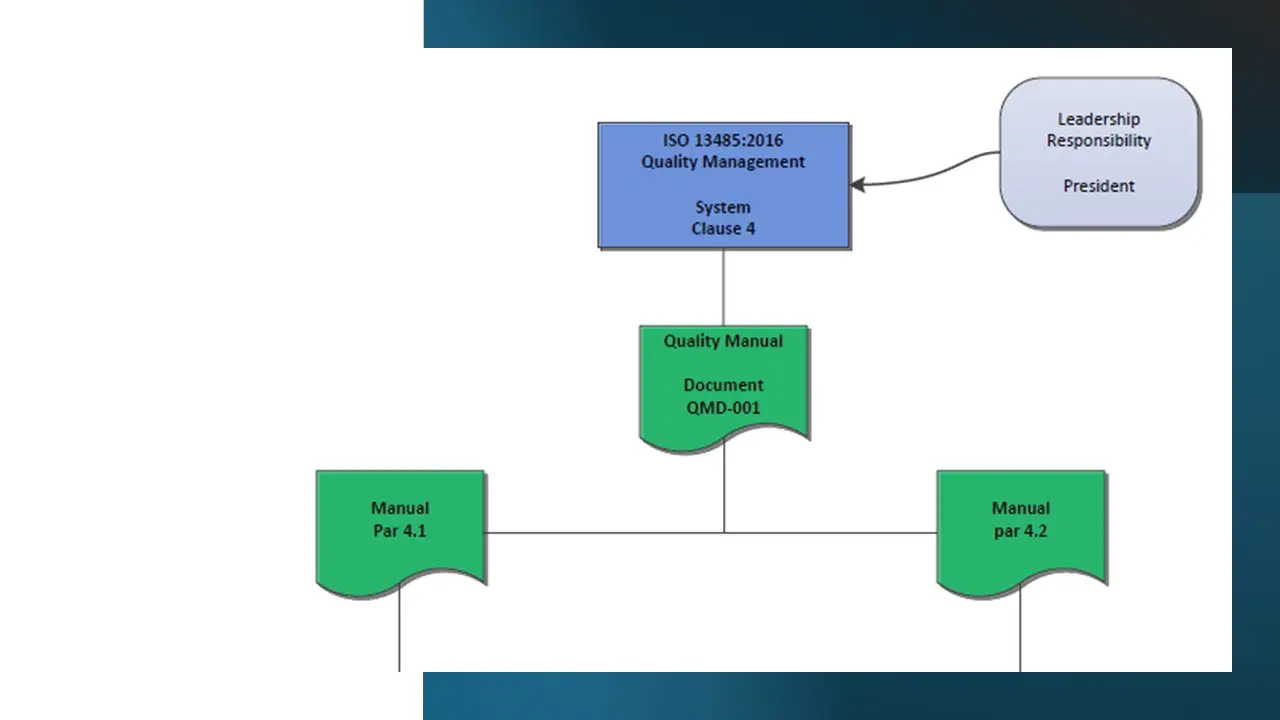
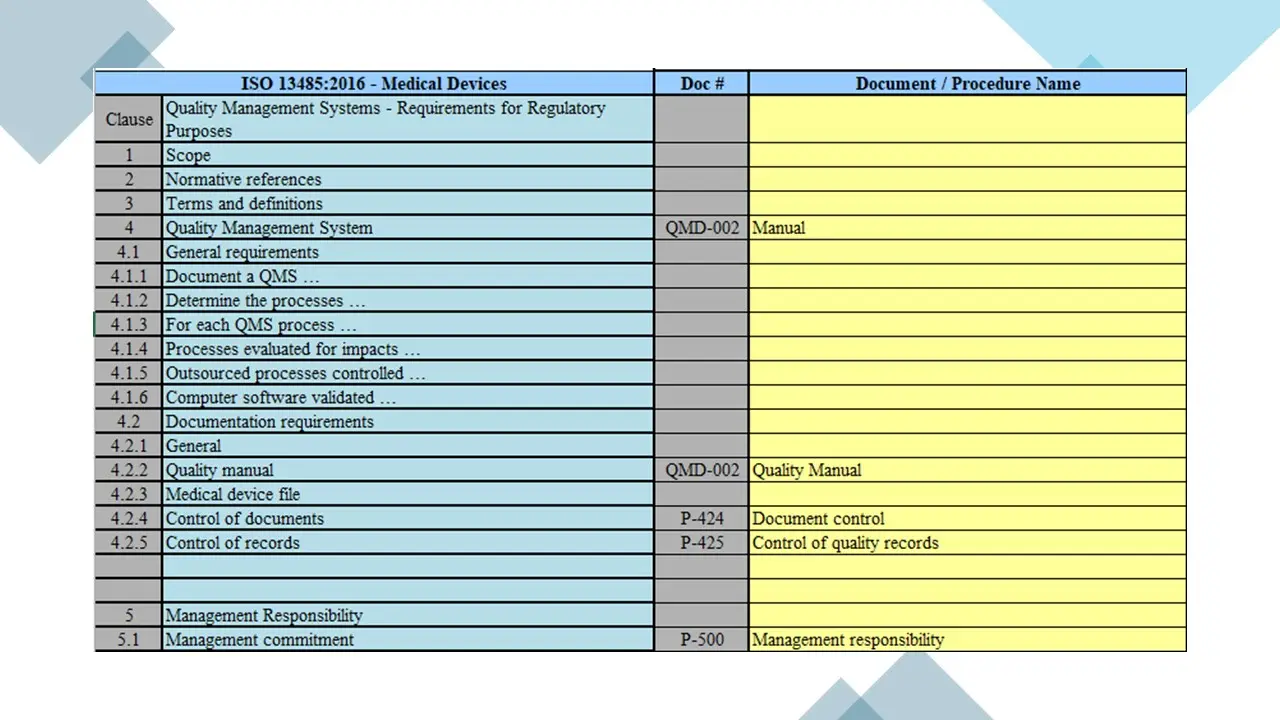
- Clause 4-Qms.Pdf
- Clause 4-Qms.Sdr
- Clause 4-Qms.Vsd
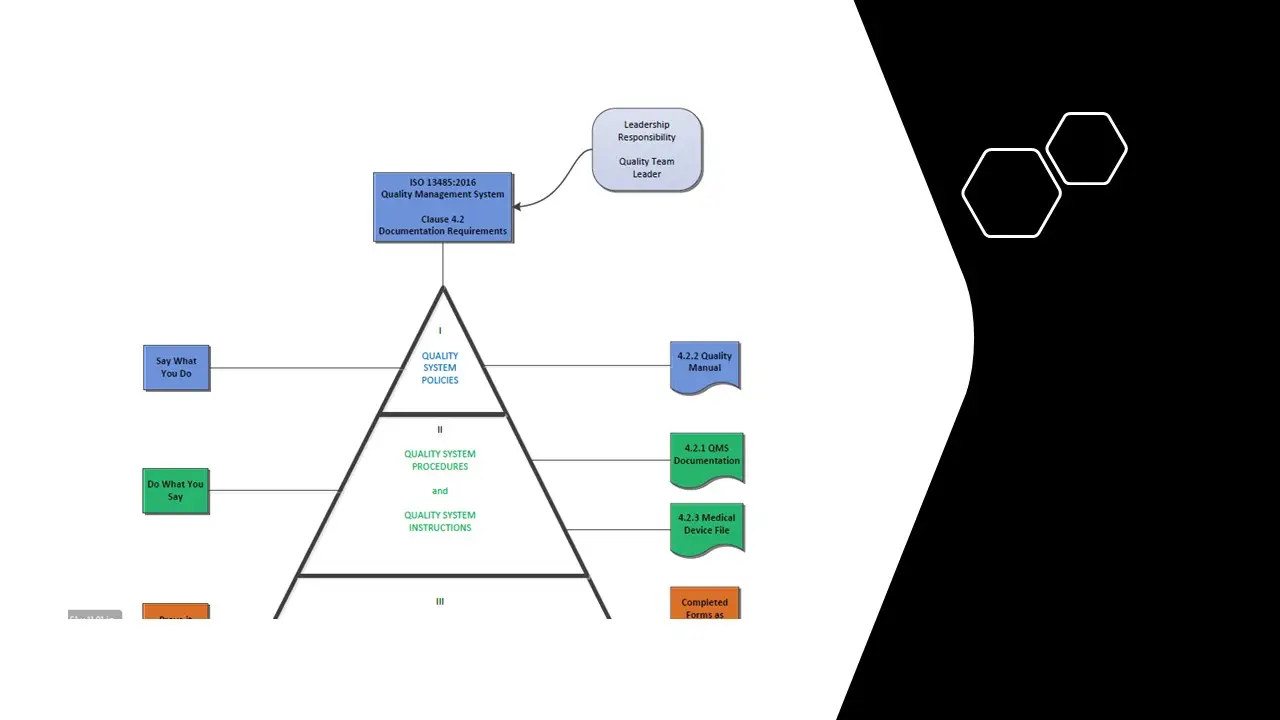
- Documentation Requirements.Pdf
- Documentation Requirements.Sdr
- Documentation Requirements.Vsd
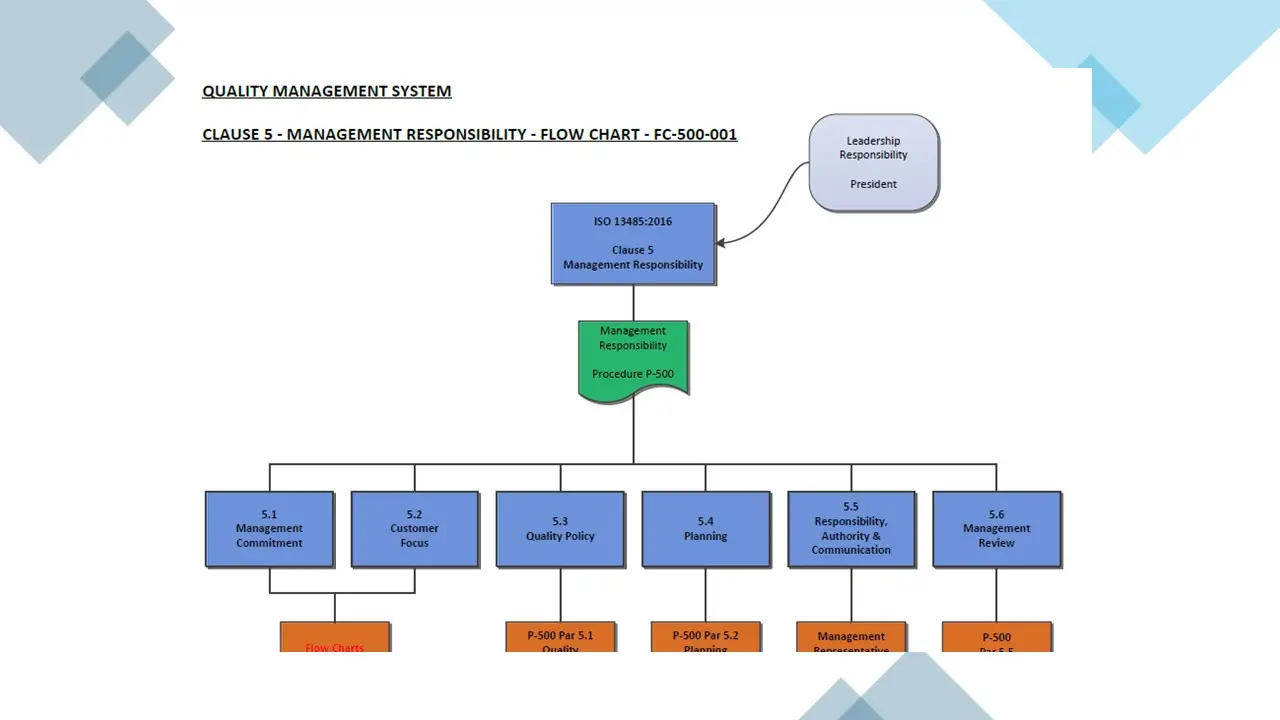
- Clause 5-Management Responsibility.Pdf
- Clause 5-Management Responsibility.Sdr
- Clause 5-Management Responsibility.Vsd
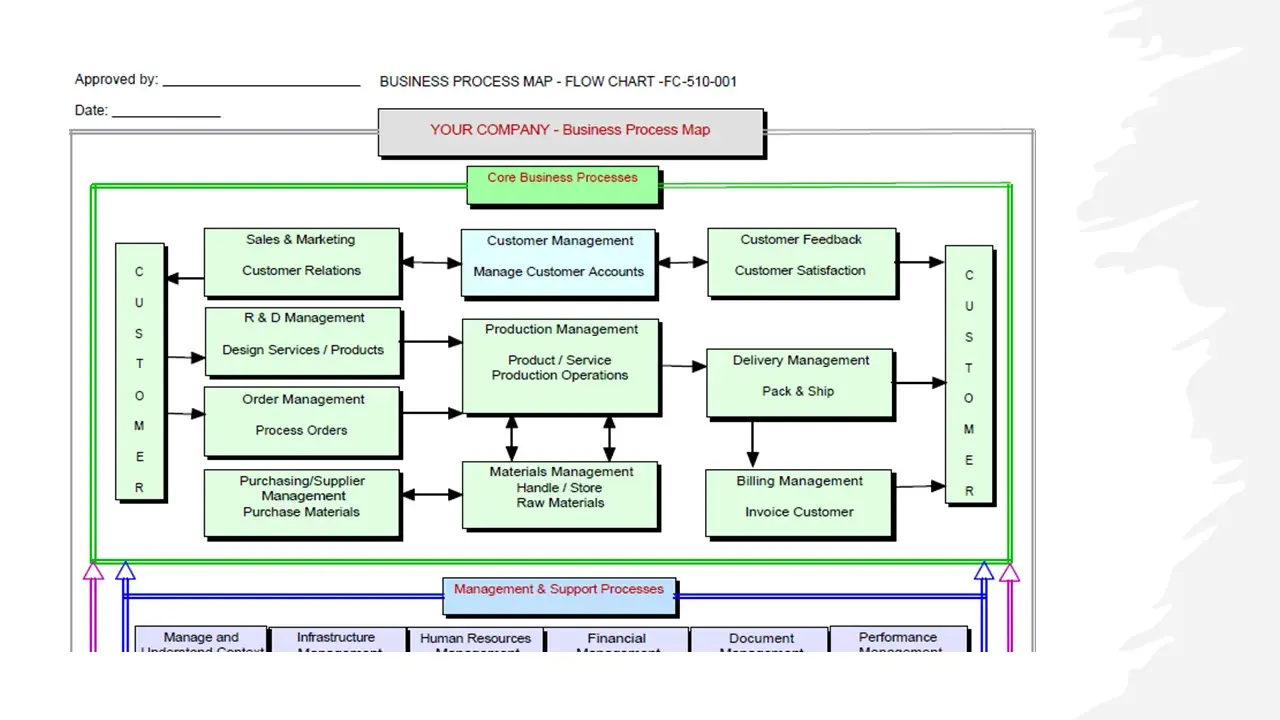
- Business Process Map.Vsd
- Business Process Map.Pdf
- Business Process Map.Sdr
- Management-Customer Focus.Vsd
- Management-Customerfocus.Pdf
- Management-Customerfocus.Sdr
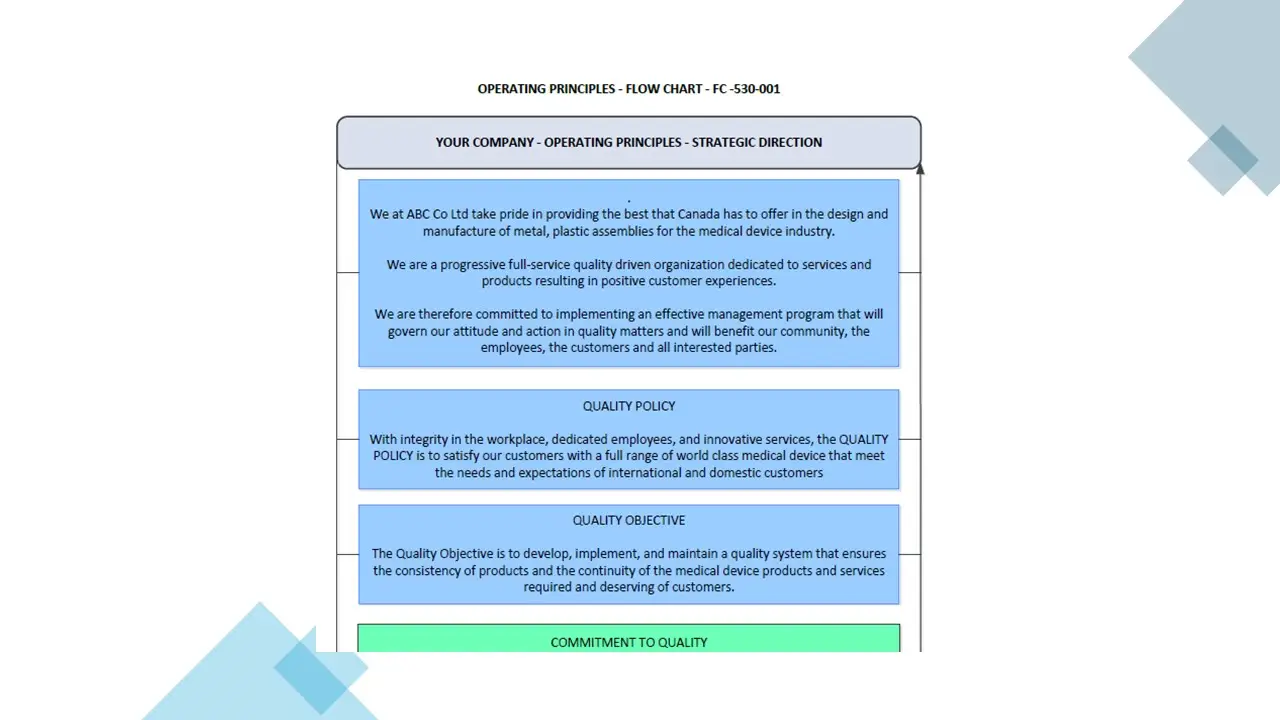
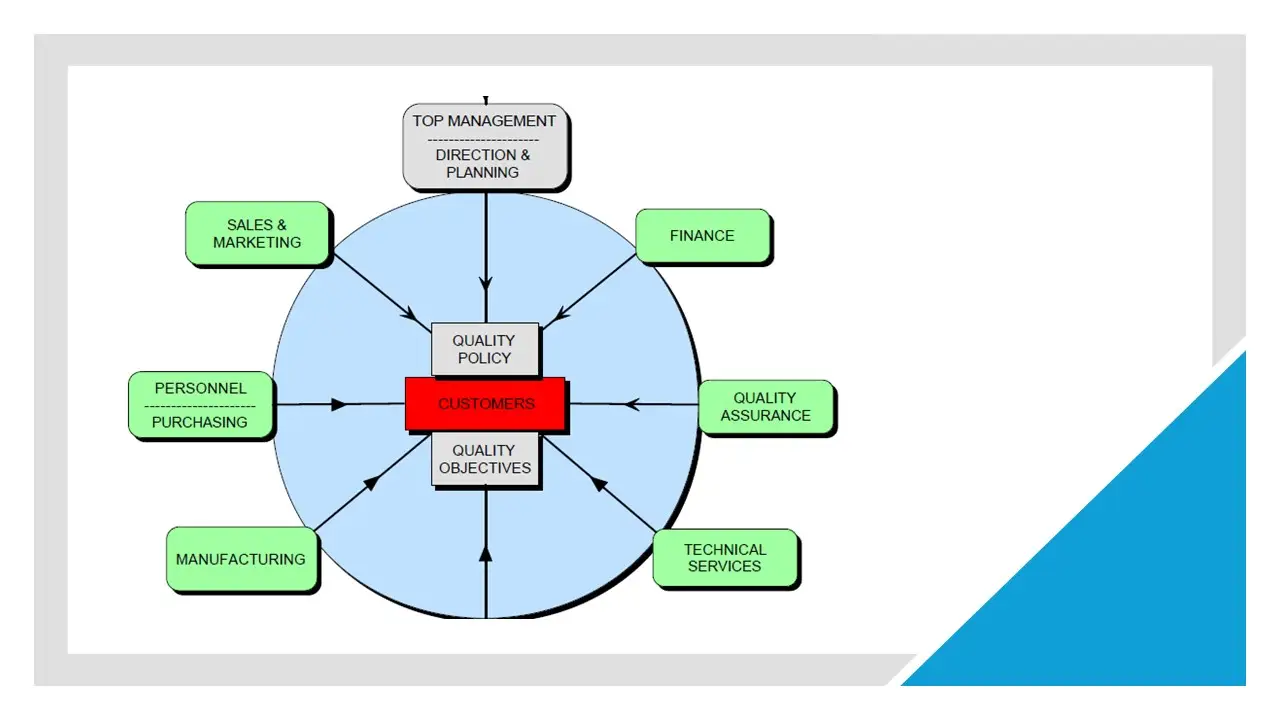
- Operating Principles.Pdf
- Operating Principles.Vsd
- Operatingprinciples.Sdr
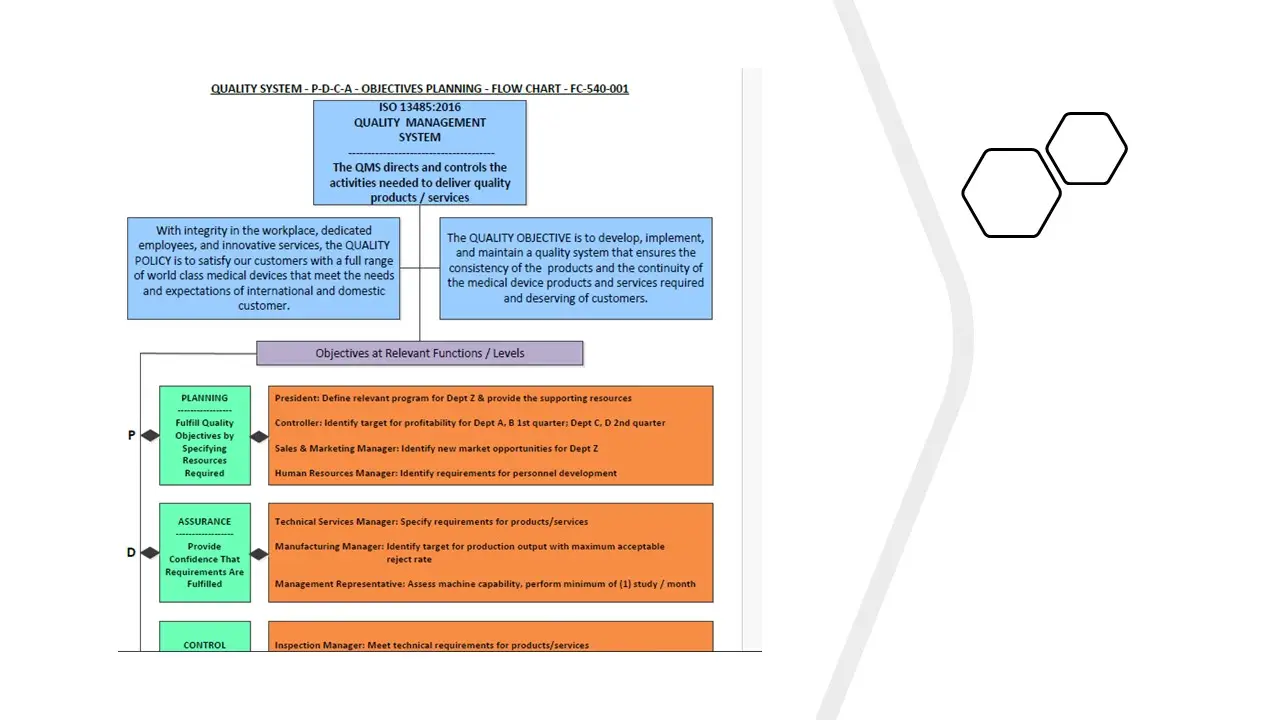
- P-D-C-A Objectives Planning.Pdf
- P-D-C-A Objectives Planning.Sdr
- P-D-C-A Objectives Planning.Vsd
- Organization-Chart.Sdr
- Organization Chart.Pdf
- Organization Chart.Vsd
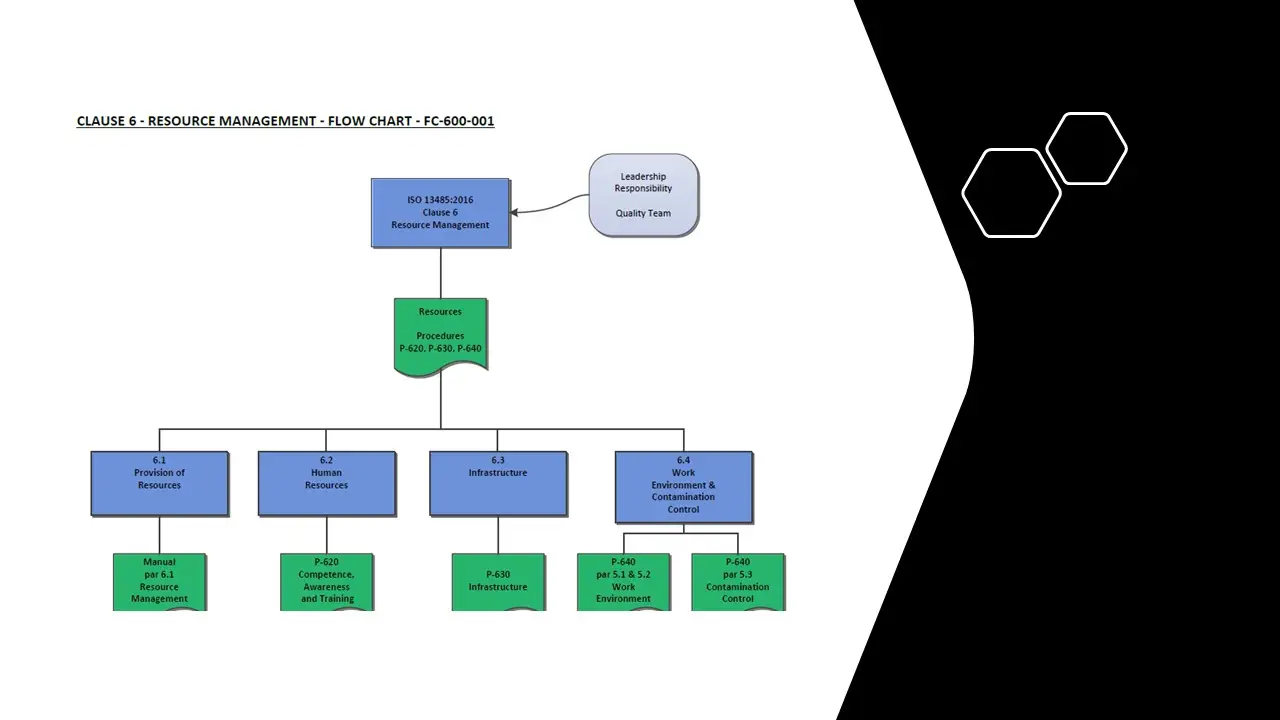
- Clause 6- Resourcemanagement.Sdr
- Clause 6-Resource Management.Pdf
- Clause 6-Resource Management.Vsd
- Contributors-5Ms.Pdf
- Contributors-5Ms.Sdr
- Contributors-5Ms.Vsd
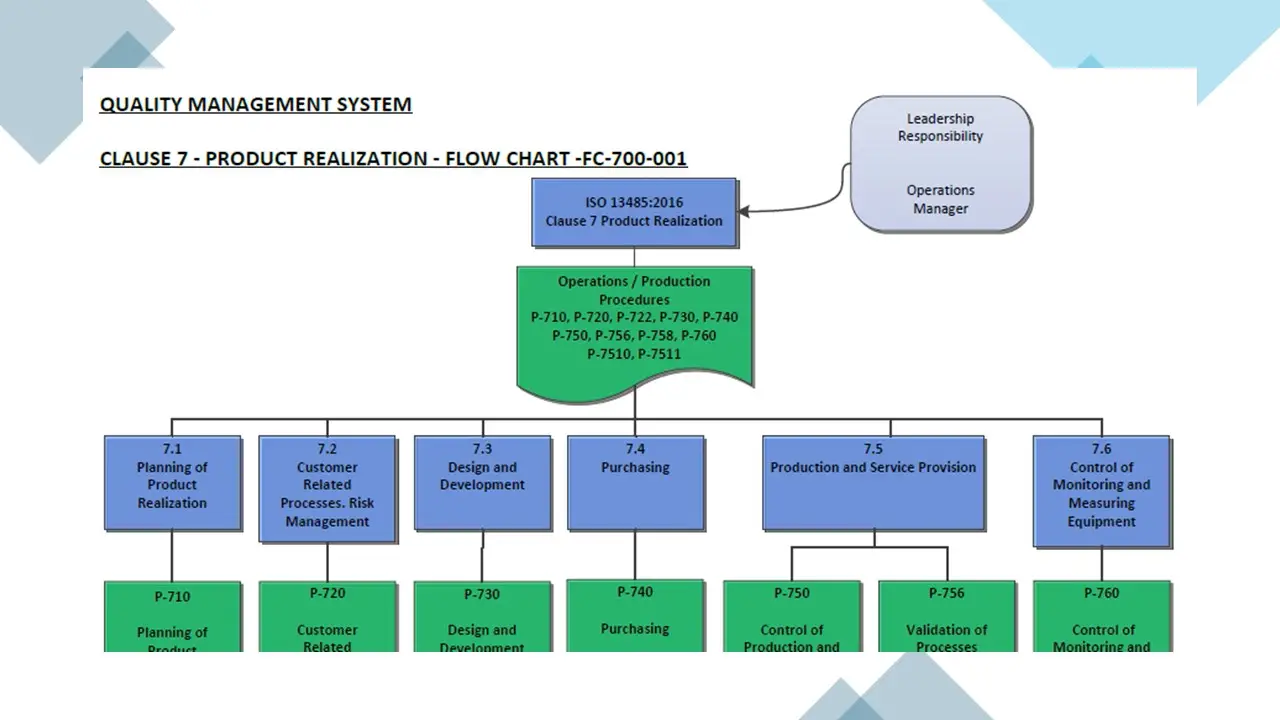
- Clause 7- Product Realization.Pdf
- Clause 7- Product Realization.Vsd
- Clause 7-Productrealization.Sdr
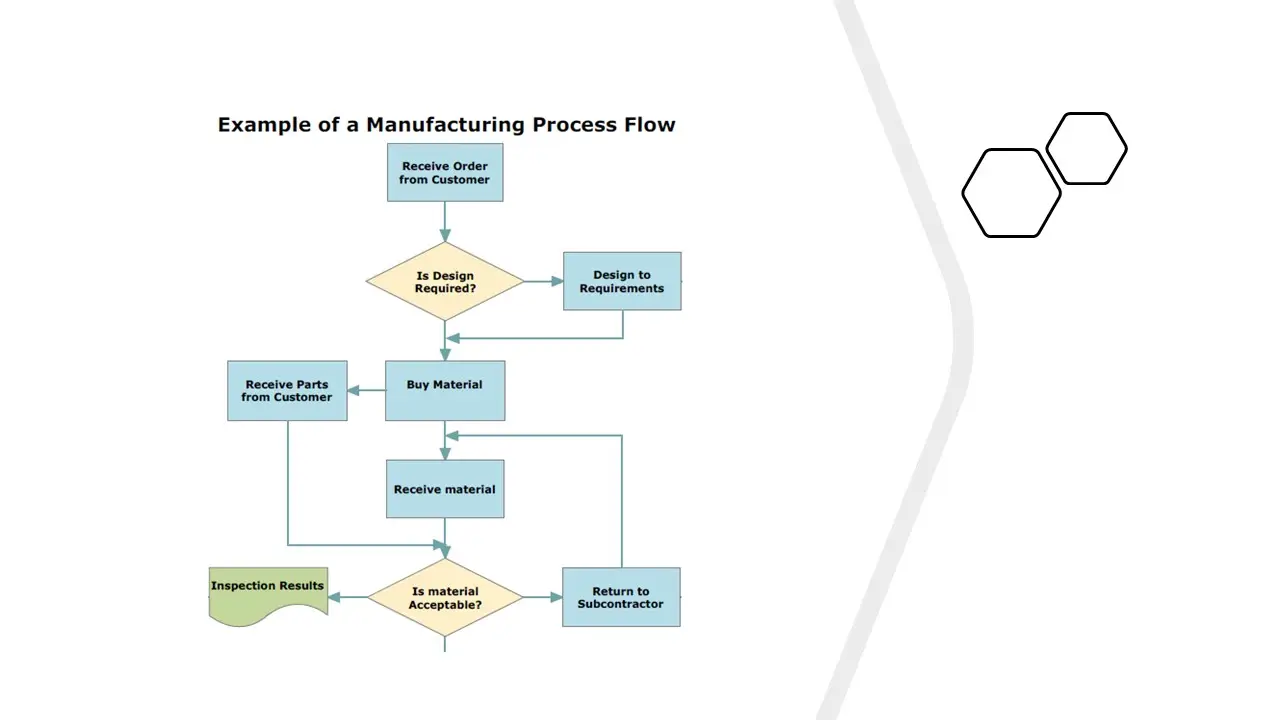
- Process Flow Chart.Pdf
- Process Flow Chart.Vsd
- Processflowchart.Sdr
- Product Realization Quality Plan.Pdf
- Product Realization Quality Plan.Vsd
- Productrealizationqualityplan.Sdr
- Customer Processes.Pdf
- Customer Processes.Vsd
- Customerprocesses.Sdr
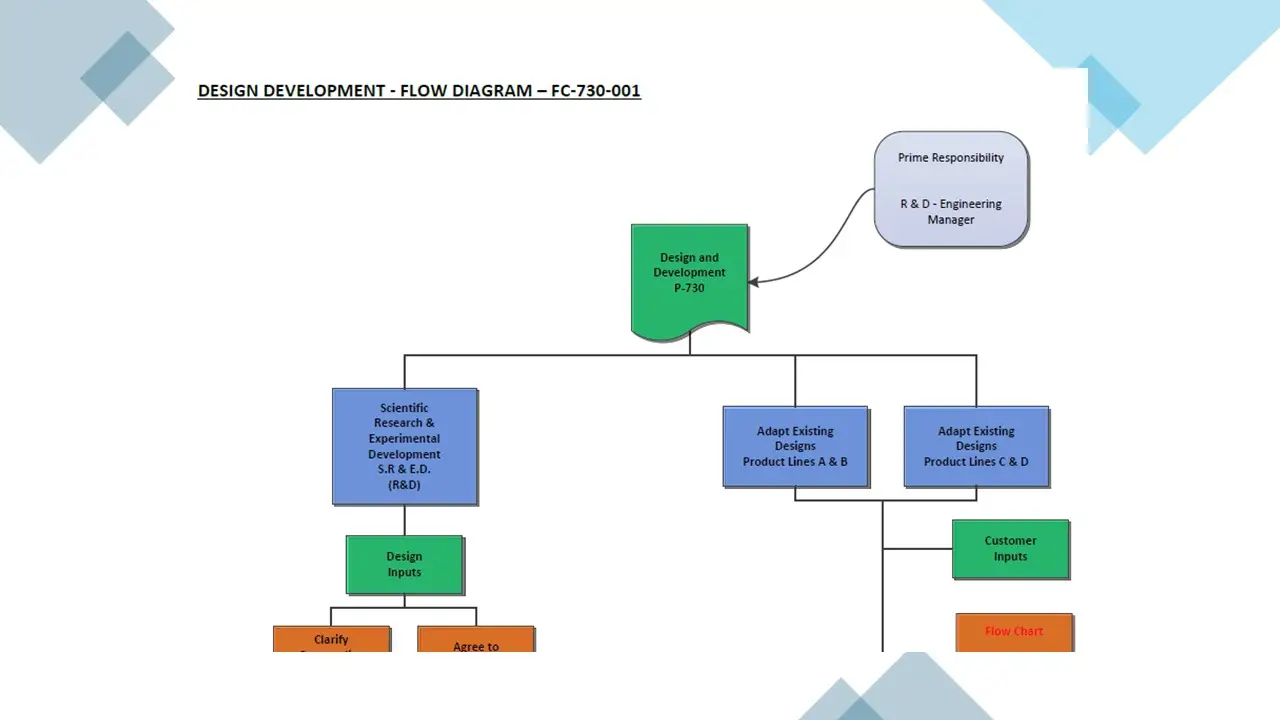
- Design Development.Pdf
- Design Development.Vsd
- Designdevelopment.Sdr
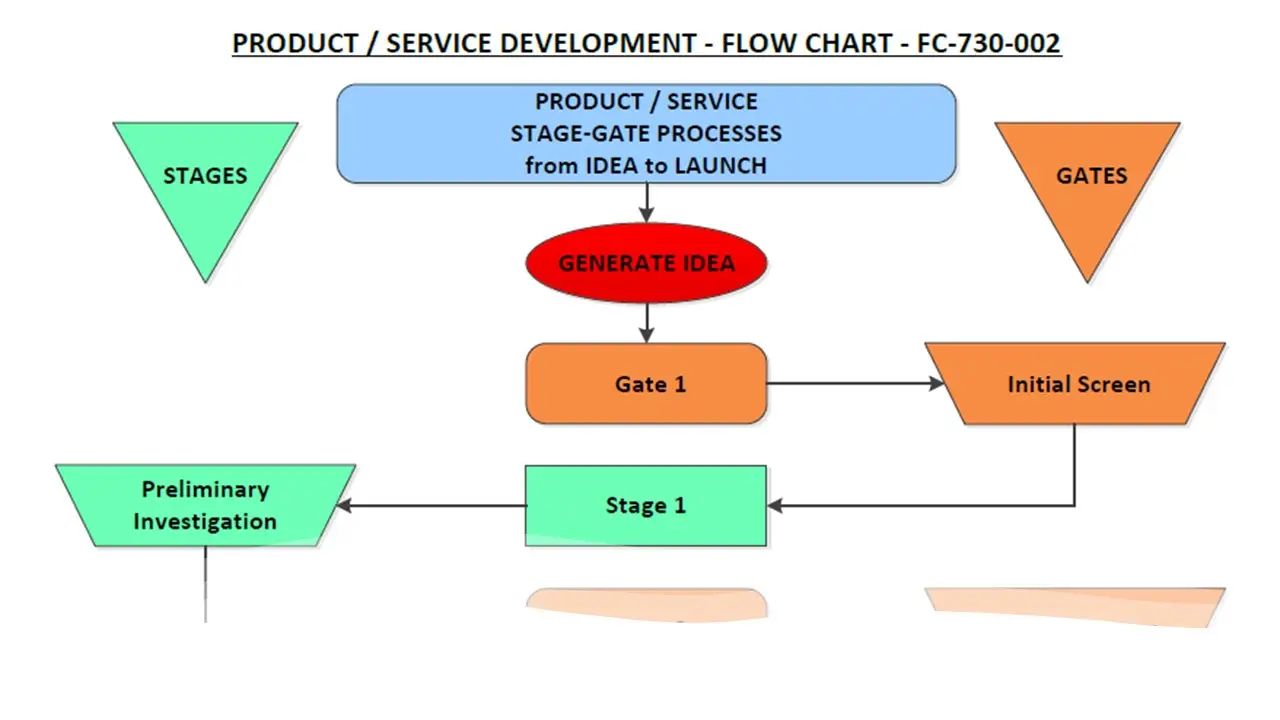
- Stage Gate Idea-To-Launch.Pdf
- Stage Gate Idea-To-Launch.Vsd
- Stagegate Idea-To-Launch.Sdr
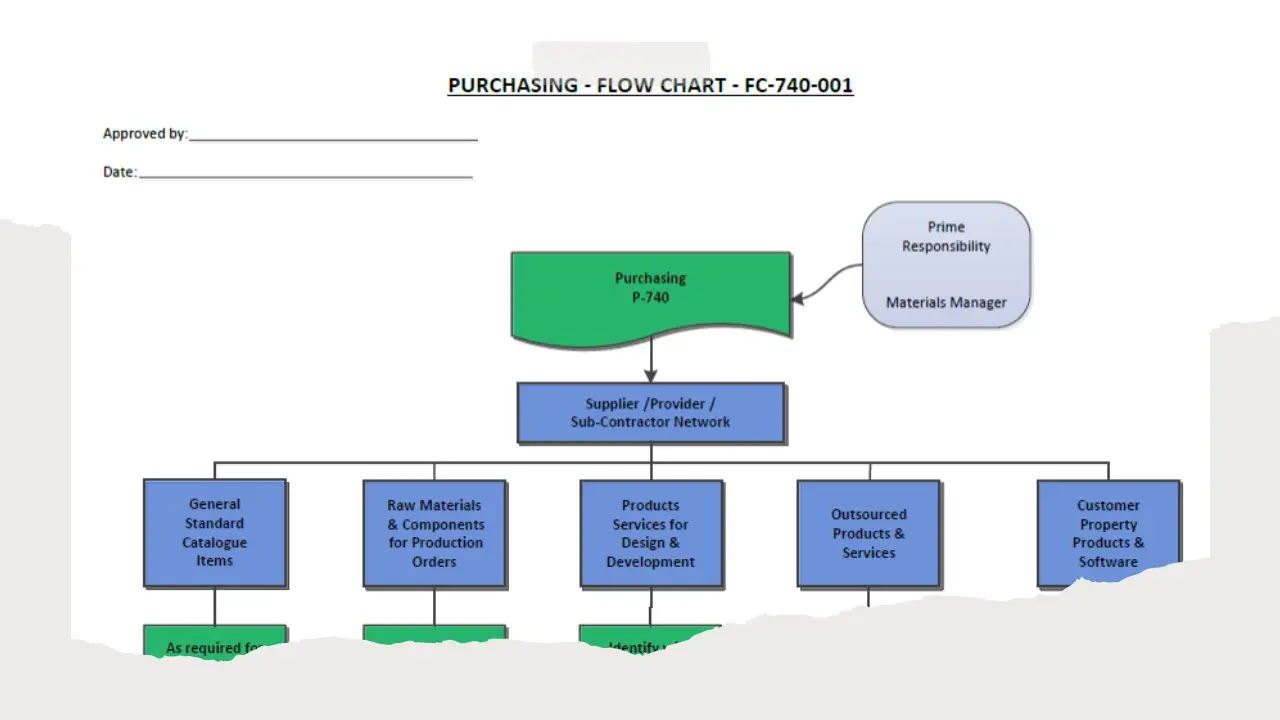
- Purchasing.Pdf
- Purchasing.Sdr
- Purchasing.Vsd
- Process Control.Pdf
- Process Control.Sdr
- Process Control.Vsd
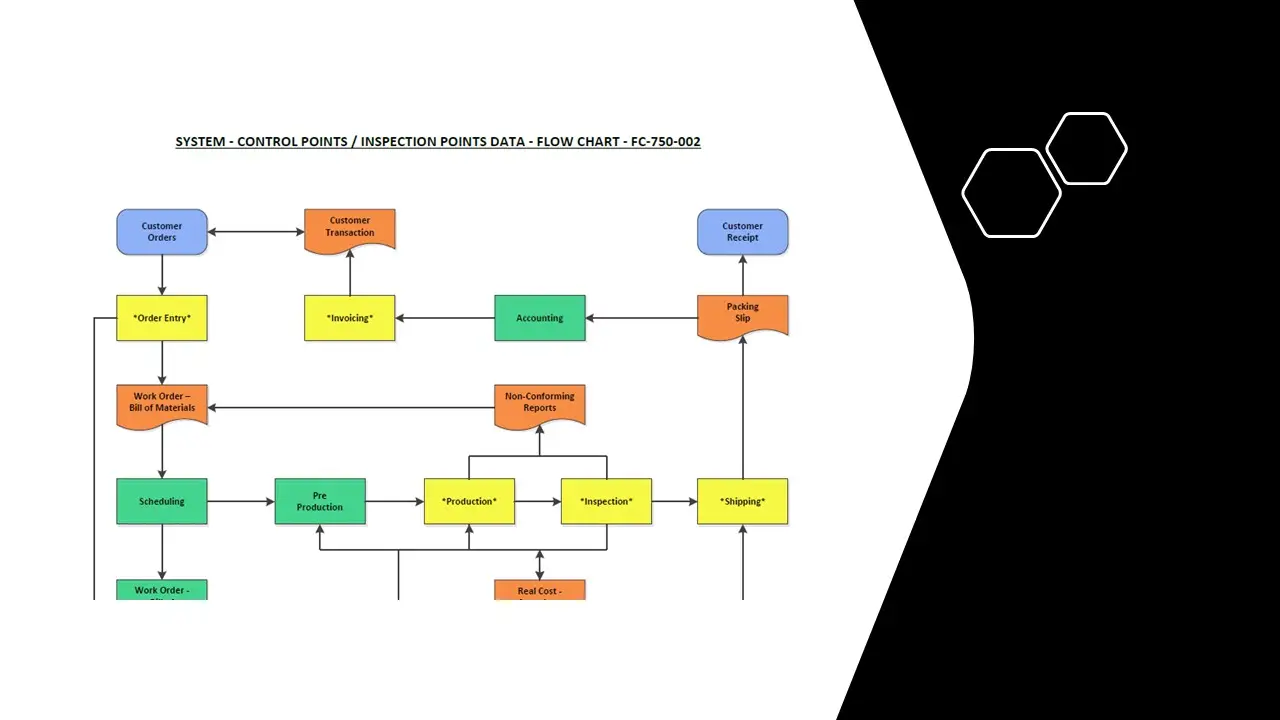
- Process Steps-Control Points.Pdf
- Process Steps-Control Points.Vsd
- Processsteps-Control Points.Sdr
- Identification Traceability.Pdf
- Identification Traceability.Vsd
- Identificationtraceability.Sdr
- Clause 8 – Measure-Improve.Pdf
- Clause 8 – Measure-Improve.Vsd
- Clause 8 -Measure-Improve.Sdr
- Continual Improvement.Pdf
- Continual Improvement.Sdr
- Continual Improvement.Vsd
- Corrective Action.Pdf
- Corrective Action.Sdr
- Corrective Action.Vsd
- 2016 Checklist.Xls
- 2016 Procedures Forms Matrix.Xls
- 2016 Quality Systems Manual.Docx
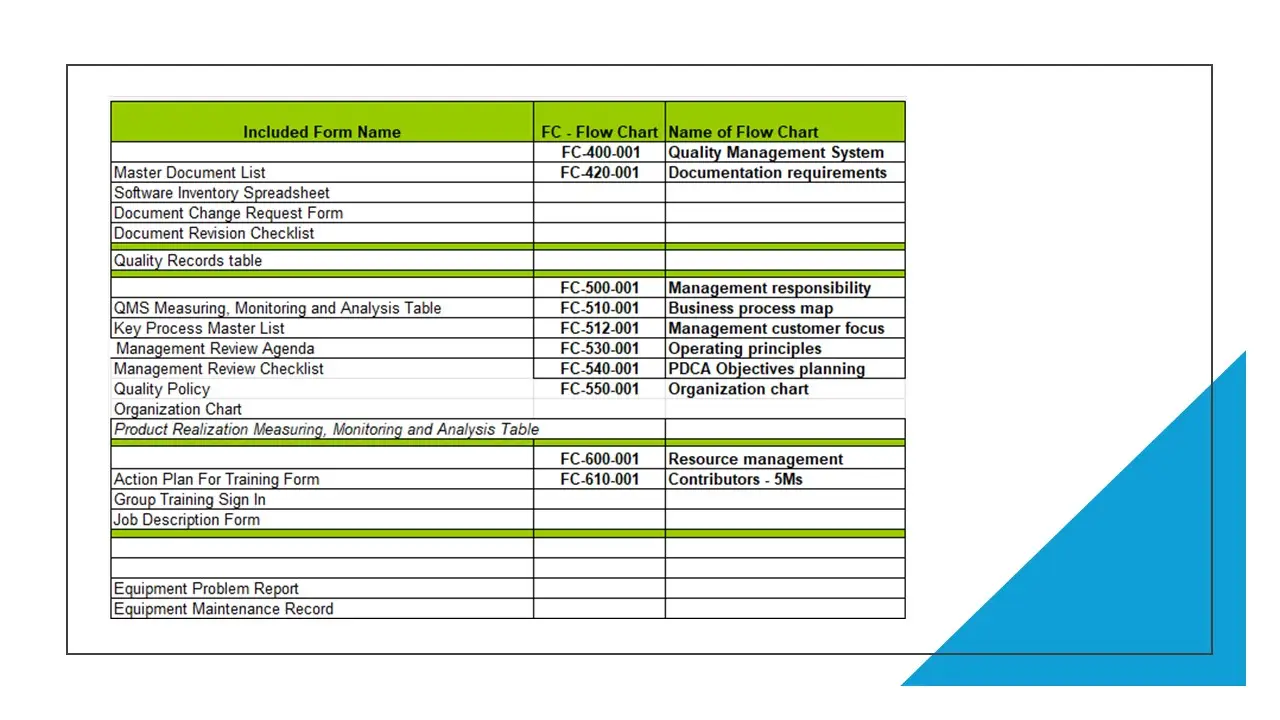
- List And Description Of Quality Management System Flow Charts.Docx
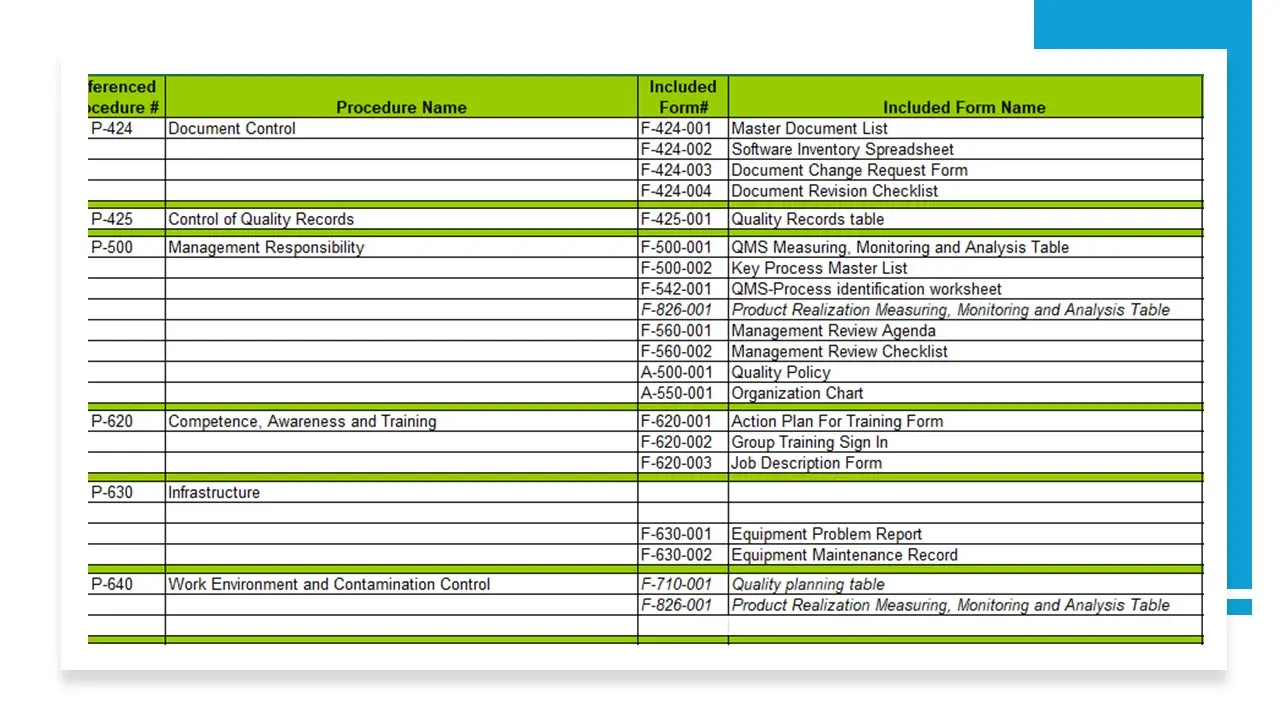
- Document Control.Doc
- Control Of Quality Records.Doc
- Management Responsibility-V2.Doc
- Competence, Awareness And Training.Doc
- Infrastructure.Doc
- Workenvironment-Contaminationcontrol.Doc
- Planning Of Product Realization Processes.Doc
- Customer Related Processes.Doc
- Risk Management.Doc
- Design And Development.Doc
- Purchasing.Doc
- Controlproductionservice-V2.Doc
- Customer Property.Doc
- Preservation Of Product.Doc
- Validation Of Processes For Product Realization.Doc
- Identification And Traceability.Doc
- Control Of Measuring And Monitoring Devices.Doc
- Post Production Feedback.Doc
- Internal Audits.Doc
- Monitoringmeasuringanalysis-Products-Processes.Doc
- Control Of Nonconforming Product.Doc
- Advisorynotices-Productrecall.Doc
- Statistical Techniques.Doc
- Root Cause Analysis.Doc
- Corrective Action.Doc
- Preventive Action.Doc
- Procedures-Forms-Fc-Matrix.Xlsx
- Procedure Template.Doc
- Work Instruction Template.Doc
- Form Template.Doc
All the docuemnts of this toolkit are developed based on ISO 13485:2016 Standard.
Hence, You just need to download and selected document and add your company name and logo.