ISO 13485:2016 Premium Toolkit
This is the most comprehensive ISO 13485:2016 document toolkit currently available.
The documents are created in Microsoft Office format and are ready to be tailored to your organization’s specific needs. As well as standard format and contents, the ISO 13485 template documents include example text that is clearly highlighted to illustrate the type of information that needs to be given regarding your organization. Full example documents are also included to help you with your implementation.
Written by a qualified auditor with over 20 years of experience in Quality Management, our toolkit provides years of experience and knowledge in an easy-to-implement format.
With quality and quantity included, this award-winning toolkit covers everything an organization will need, so you can use it first to become certified to the standard, and then to develop and continually improve your Medical Devices Quality Management System.
What is included within the ISO 13485:2016 toolkit?
- 140+ template documents – including policies, procedures, controls, checklists, tools, and other useful documentation
- Available as an instant download after purchase
140+ Templates
Medical Devices Quality Management System Documentation pack
A full and comprehensive documentation pack to help clients, consultants and service providers achieve ISO 9001:2015 successfully.
Pack folder structure:
- QMS-FlowCharts
- QMS-Forms
- QMS-Procedures
- QMS-Quality-Manual
List of all documents:
- Quality Policy.Doc
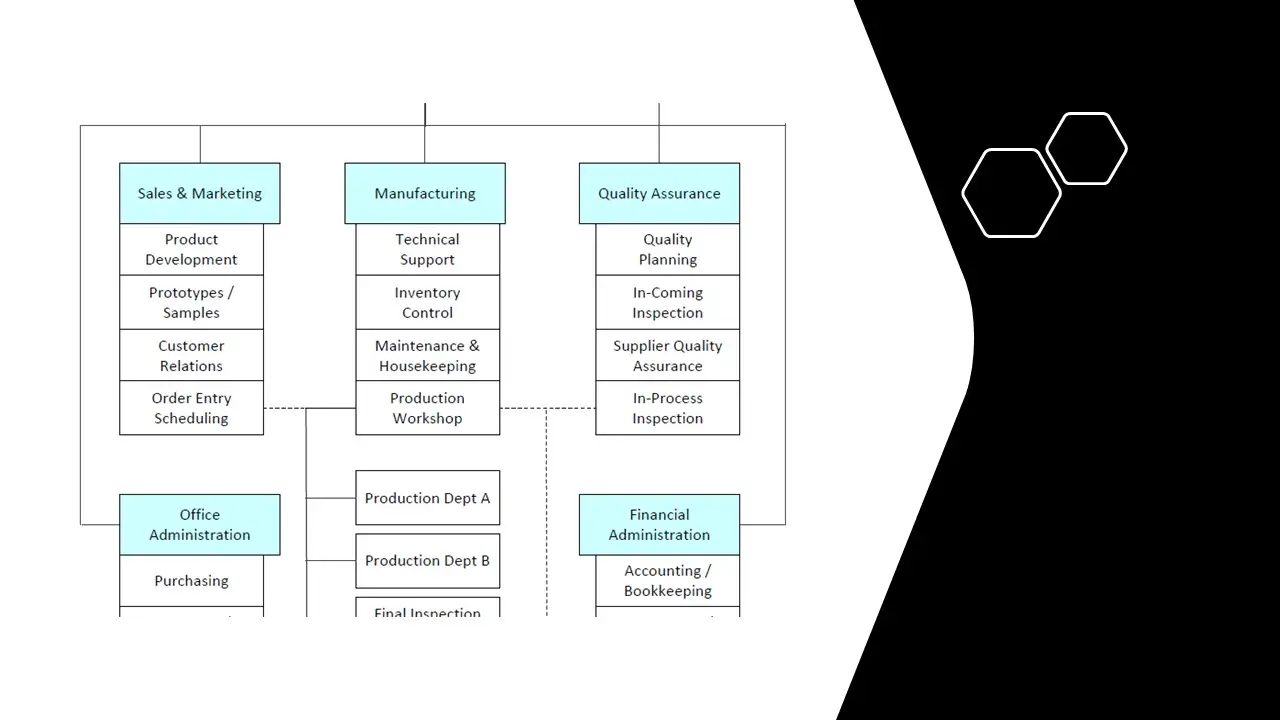
- Organization Chart.Doc
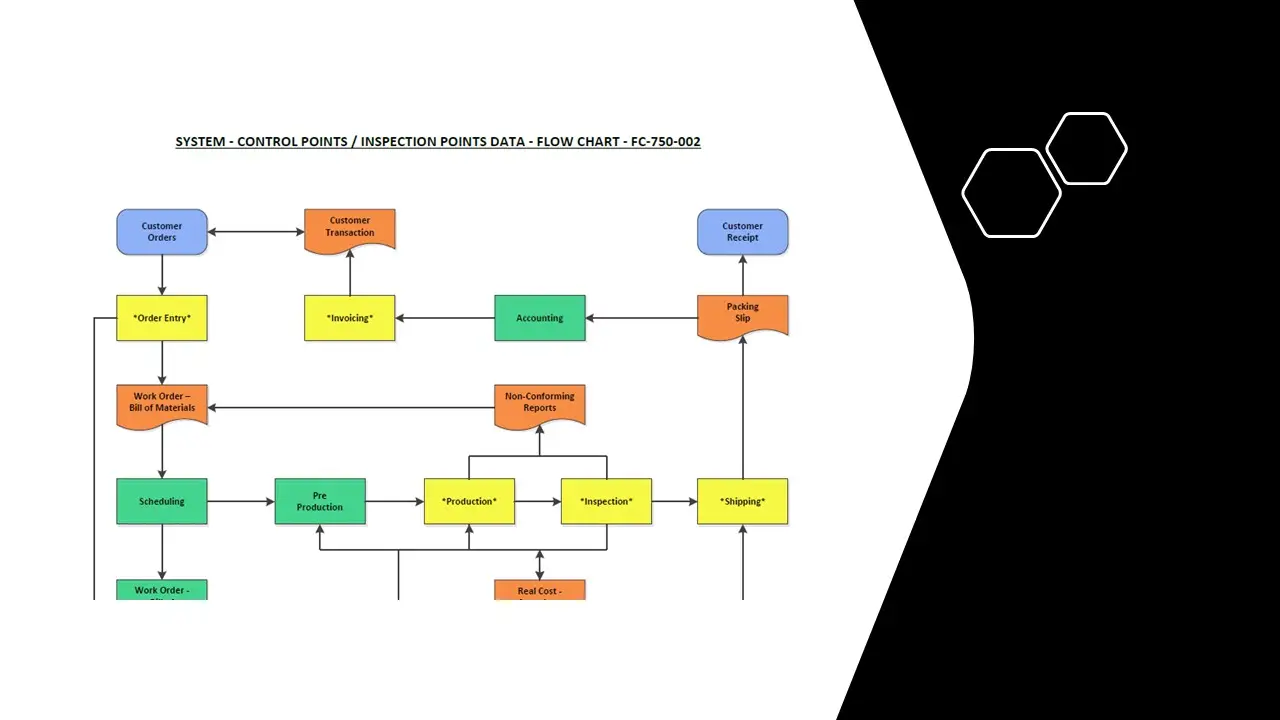
- Flow Chart For Iso Qm.Pdf
- Software Inventory.Doc
- Document Change Request.Doc
- Document Revision Checklist.Doc
- Quality Records Table-V2.Doc
- Qms Measuring Monitoring And Analysis Table.Doc
- Key Process Master List.Doc
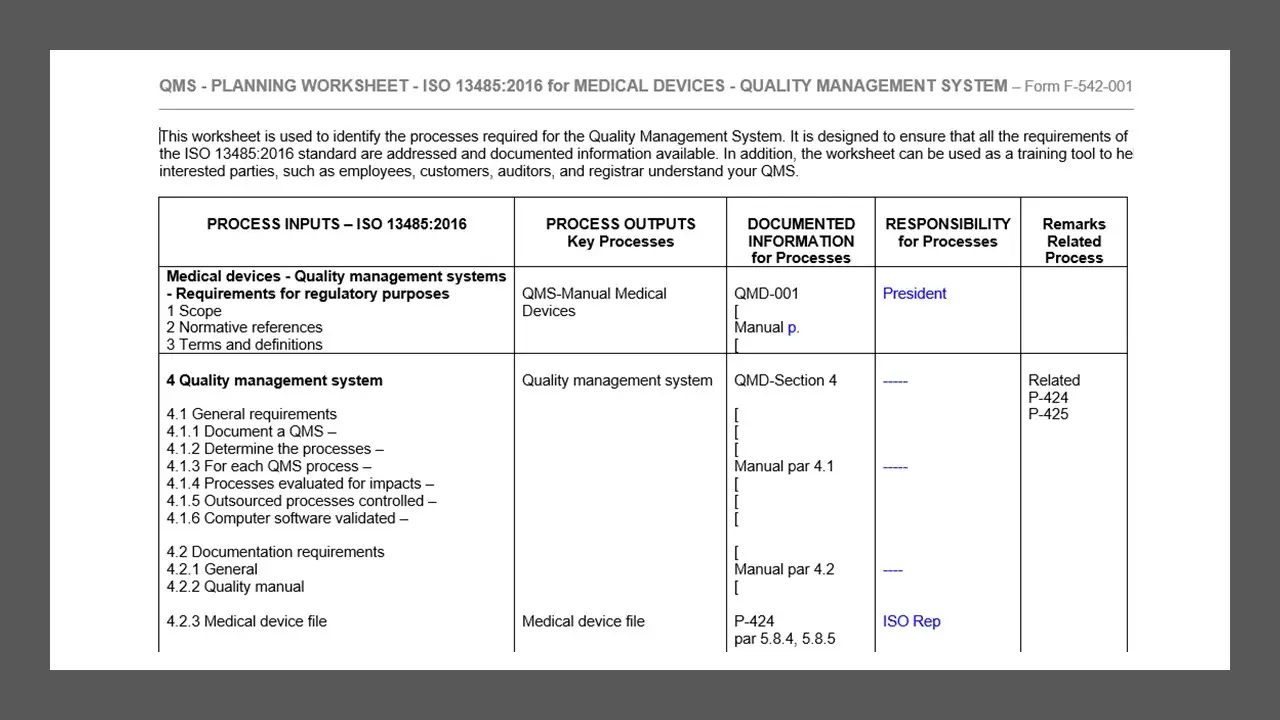
- Qms-Planning-Worksheet.Doc
- Management Review Agenda.Doc
- Management Review Checklist.Doc
- Action Plan For Training-Form.Doc
- Group Training Sign-In.Doc
- Job Description Form.Doc
- Equipment Problem Report.Doc
- Equipment Maintenance Record.Doc
- Clause-Inclusionexclusion Worksheet.Doc
- Quality Planning Table-Form.Doc
- Client Assessment Memo.Doc
- Risk Management Plan.Doc
- Risk List.Doc
- Design Plan.Doc
- Design Review.Doc
- Design Change Form.Doc
- Supplier Quality Report.Doc
- Supplier Corrective Action Request.Doc
- Approved Subcontractor List.Doc
- Process Routing Summary Sheet.Doc
- Process Routing Detail Sheet.Doc
- Customer Property Control Log.Doc
- Storage Inspection Report.Doc
- Process Validation Worksheet.Doc
- Traceability Serial Number Log.Doc
- Equipment List.Xls
- Customer Satisfaction Survey.Doc
- Audit Plan.Doc
- Internal Audit Report.Doc
- Procedure By Work Area.Doc
- Audit Checklist.Doc
- Product Realization Measuring Monitoring And Analysis Table.Doc
- Rejected Material-Disposition Report-Ncr.Doc
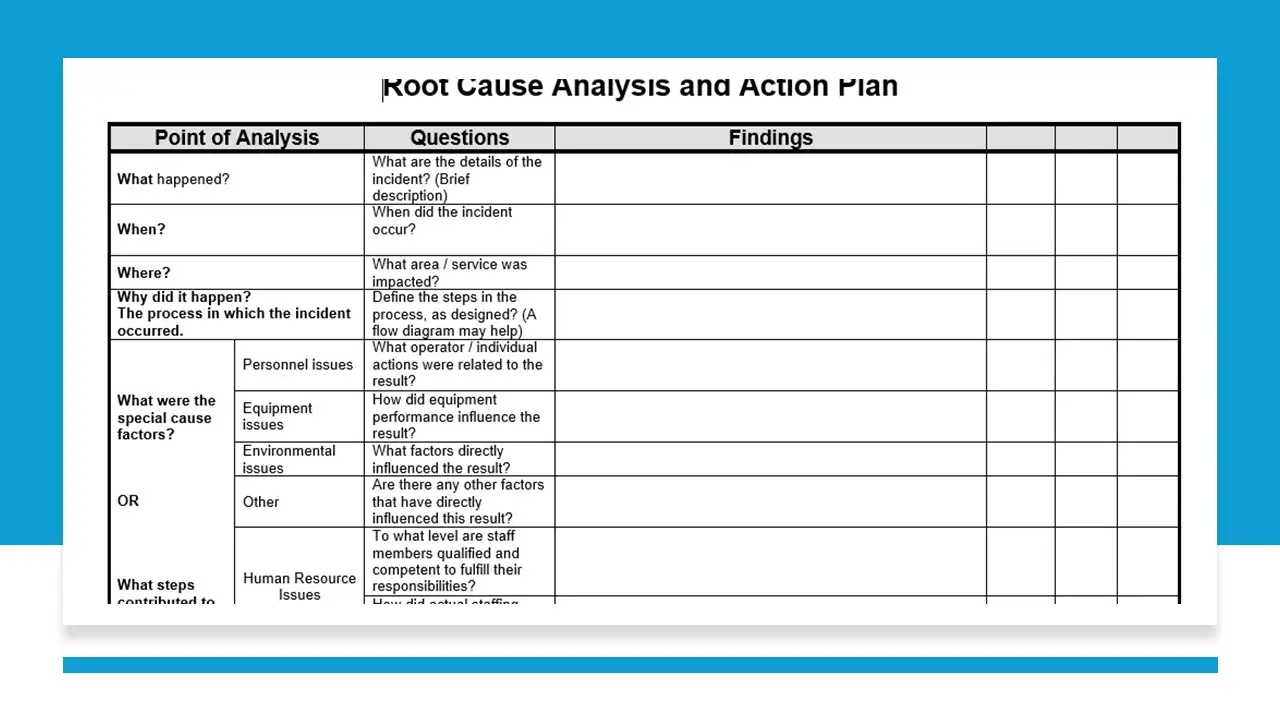
- Root Cause Analysis Action Plan.Doc
- Corrective Preventive Action Form.Doc
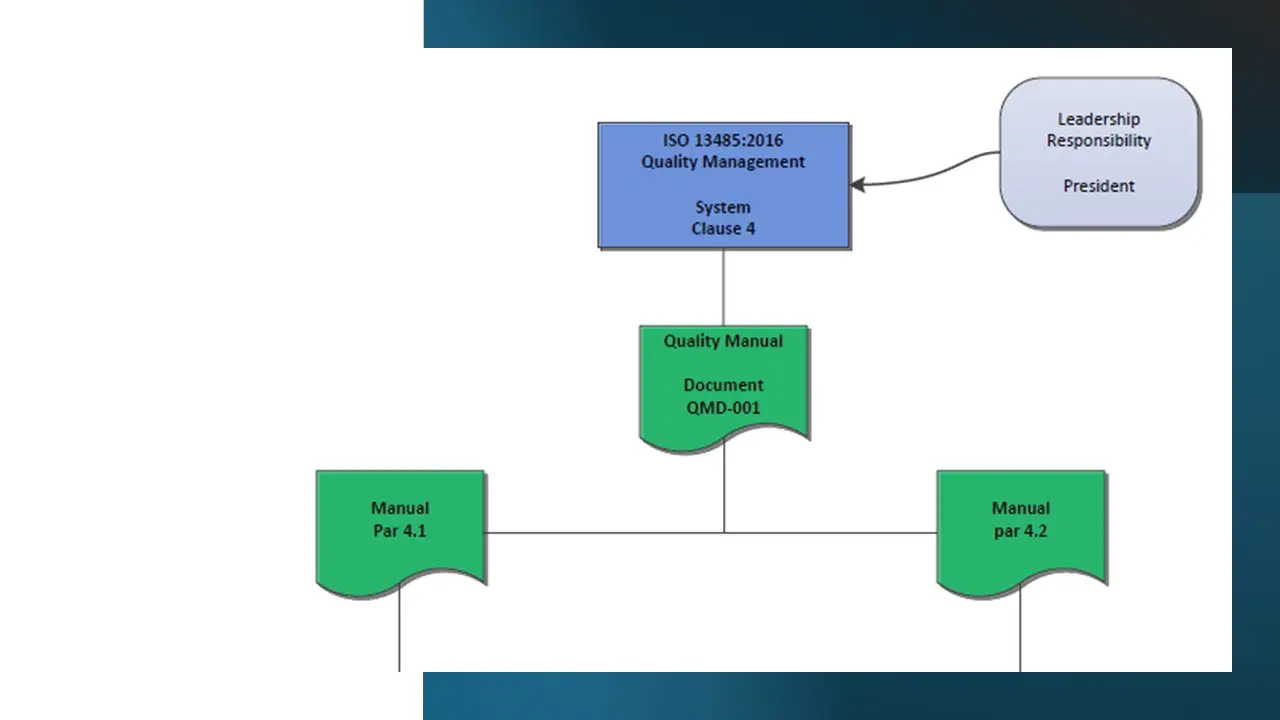
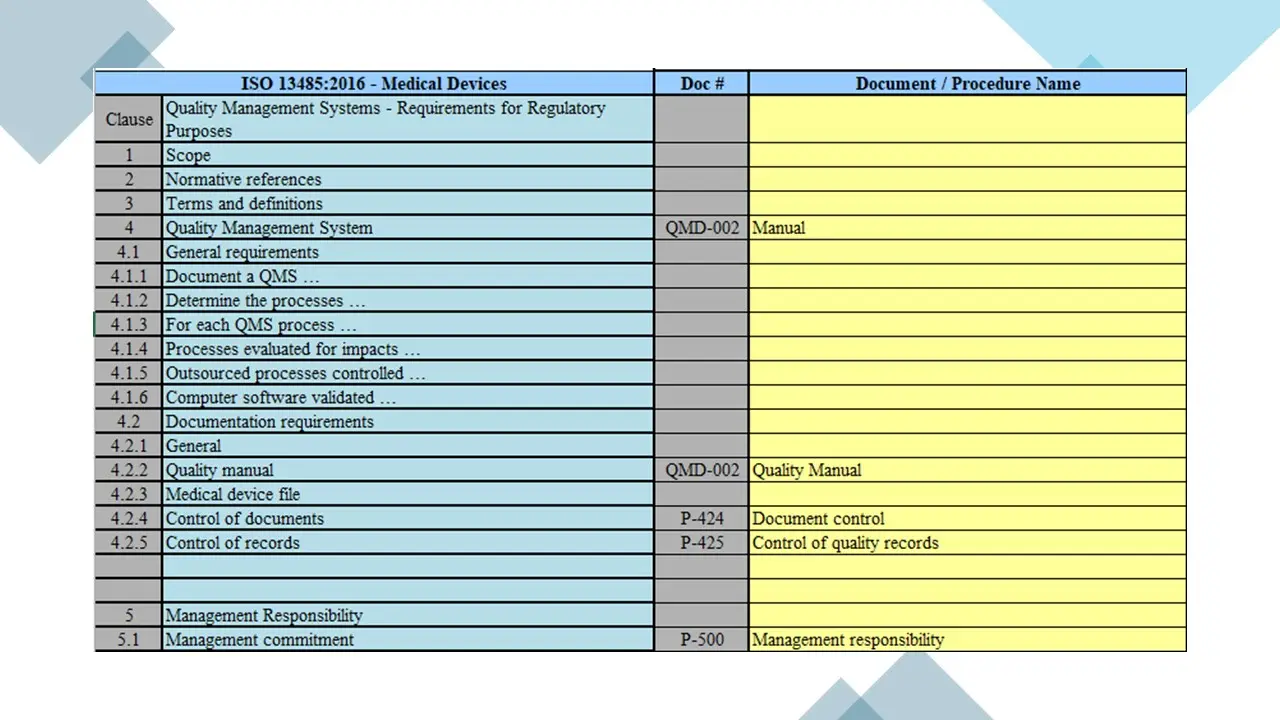
- Clause 4-Qms.Pdf
- Clause 4-Qms.Sdr
- Clause 4-Qms.Vsd
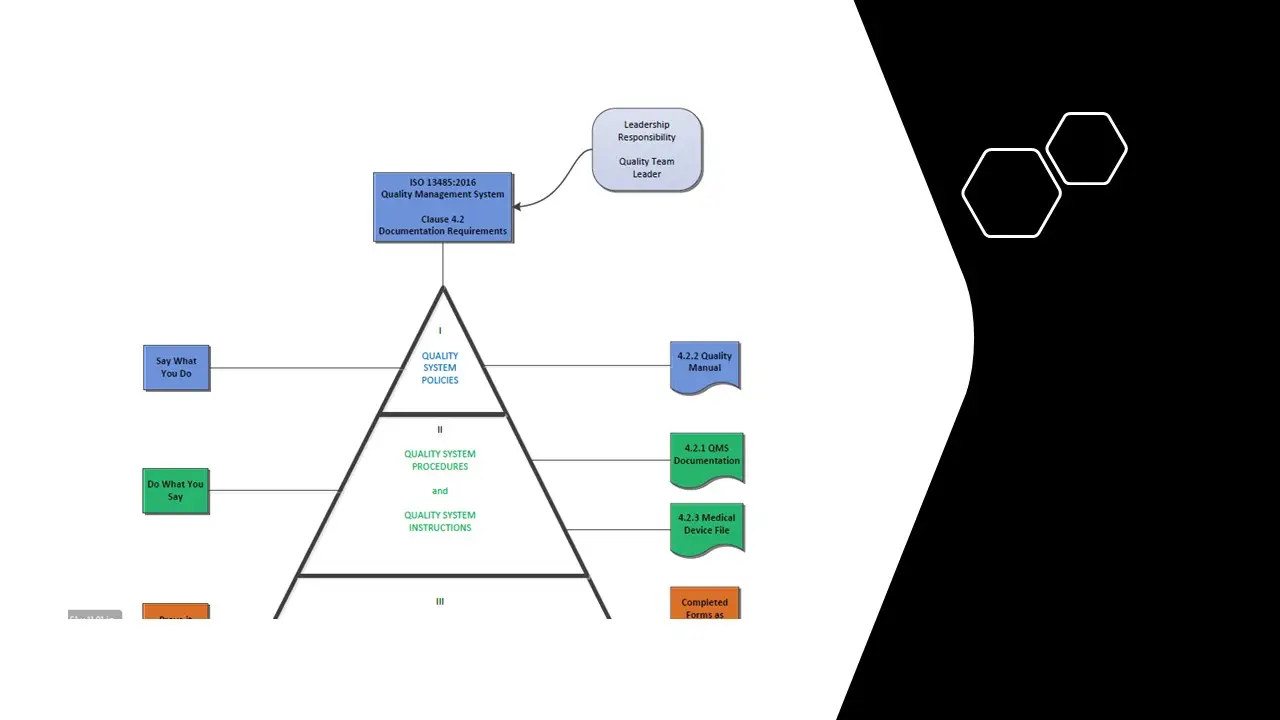
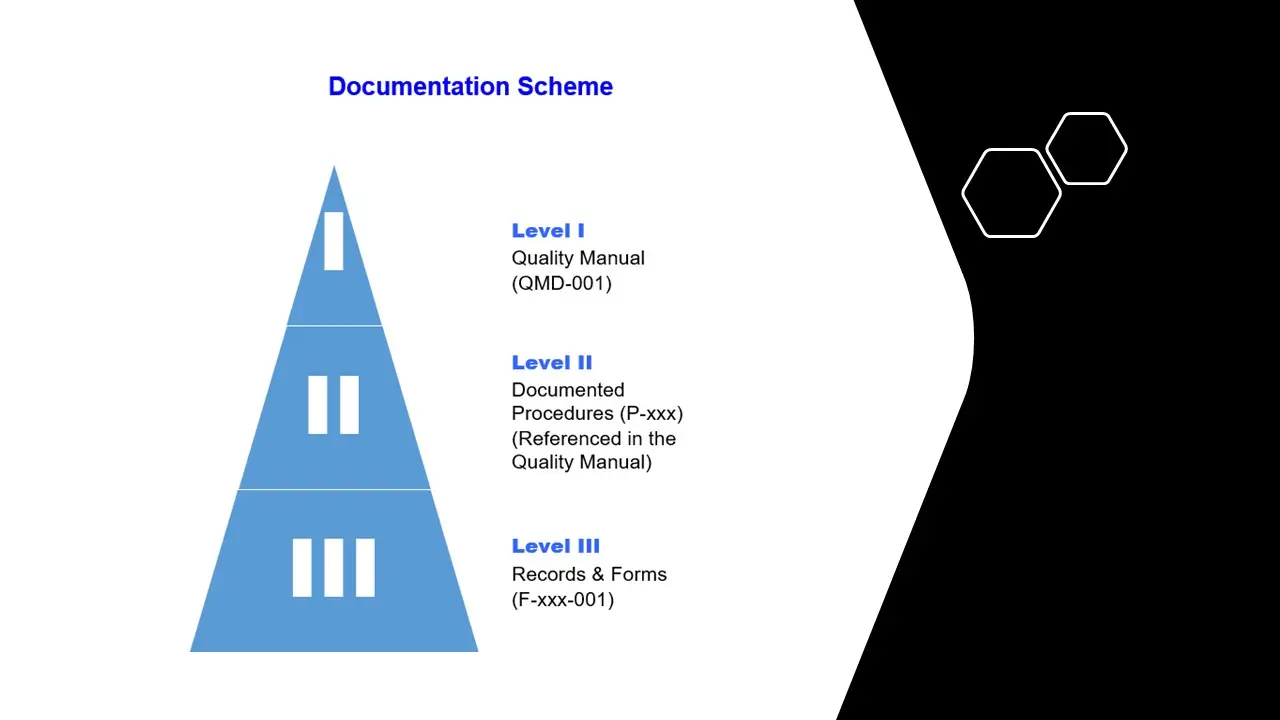
- Documentation Requirements.Pdf
- Documentation Requirements.Sdr
- Documentation Requirements.Vsd
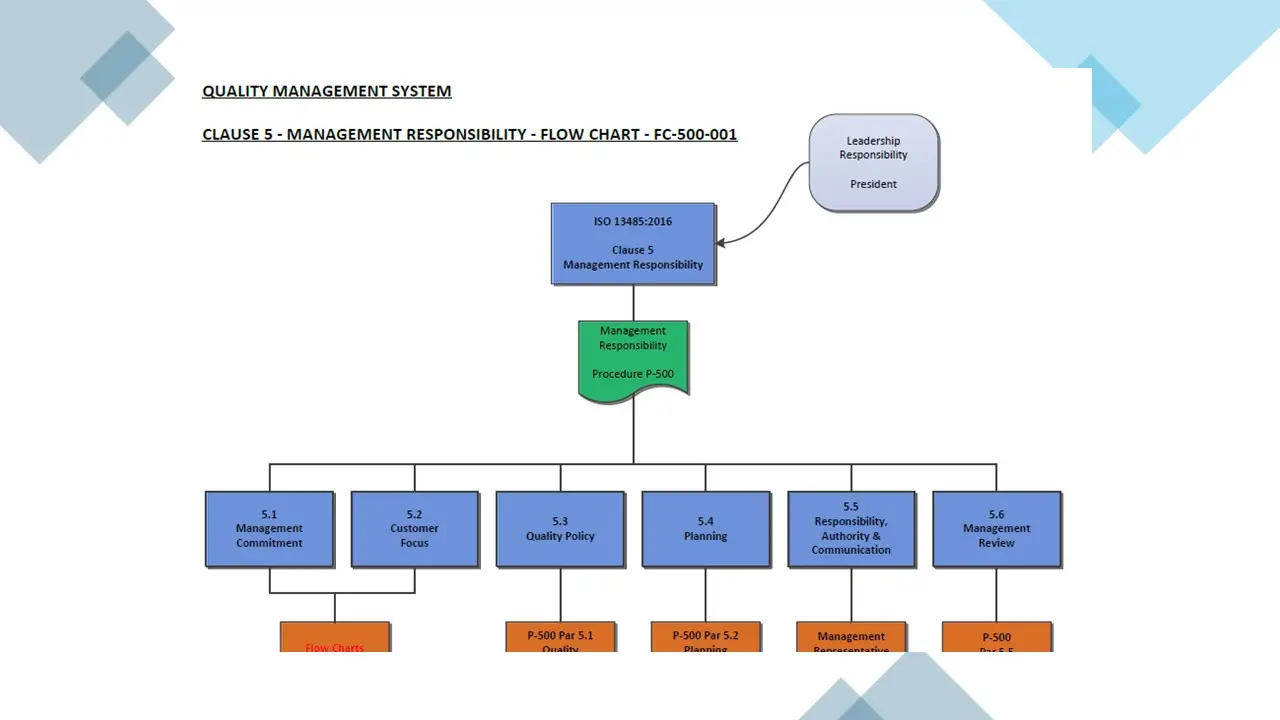
- Clause 5-Management Responsibility.Pdf
- Clause 5-Management Responsibility.Sdr
- Clause 5-Management Responsibility.Vsd
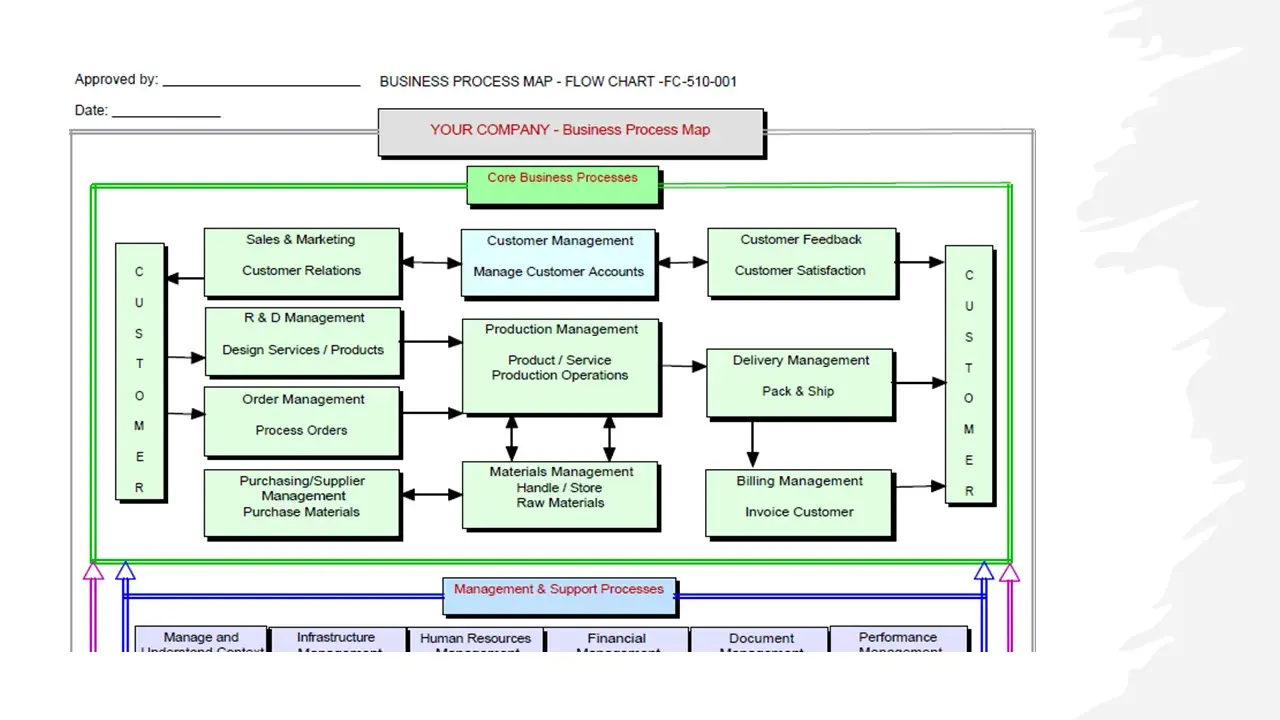
- Business Process Map.Vsd
- Business Process Map.Pdf
- Business Process Map.Sdr
- Management-Customer Focus.Vsd
- Management-Customerfocus.Pdf
- Management-Customerfocus.Sdr
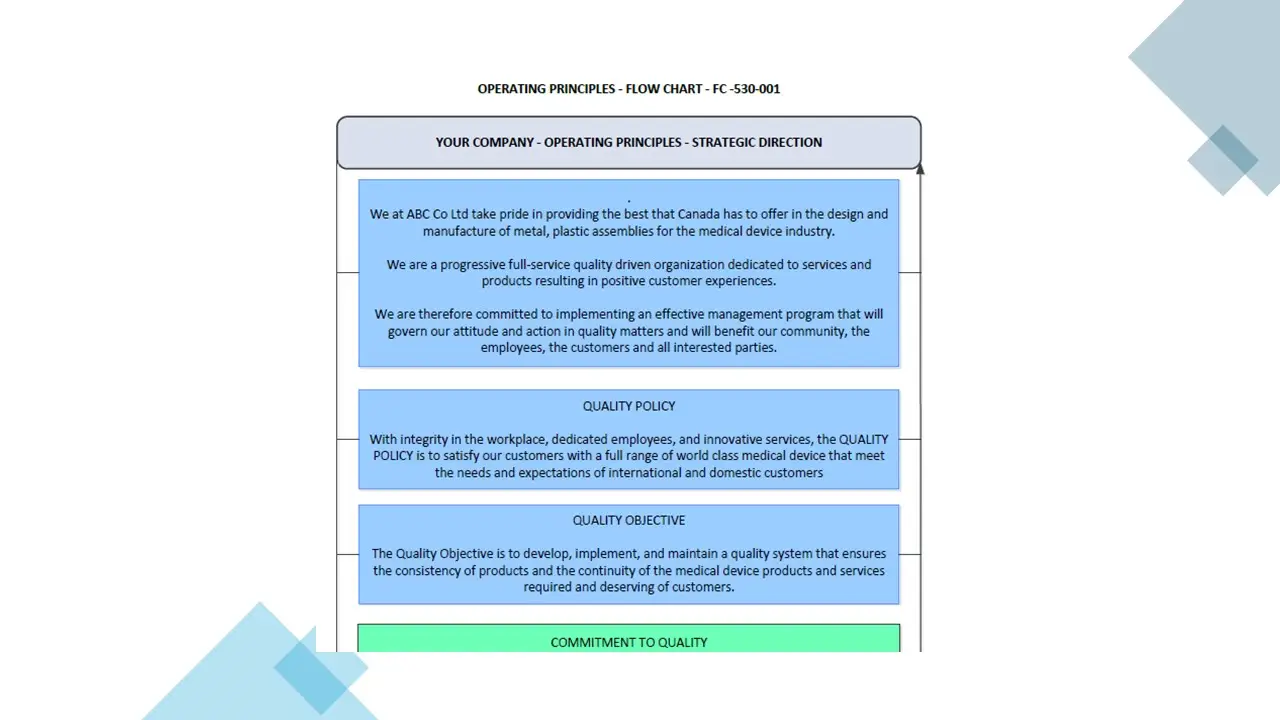
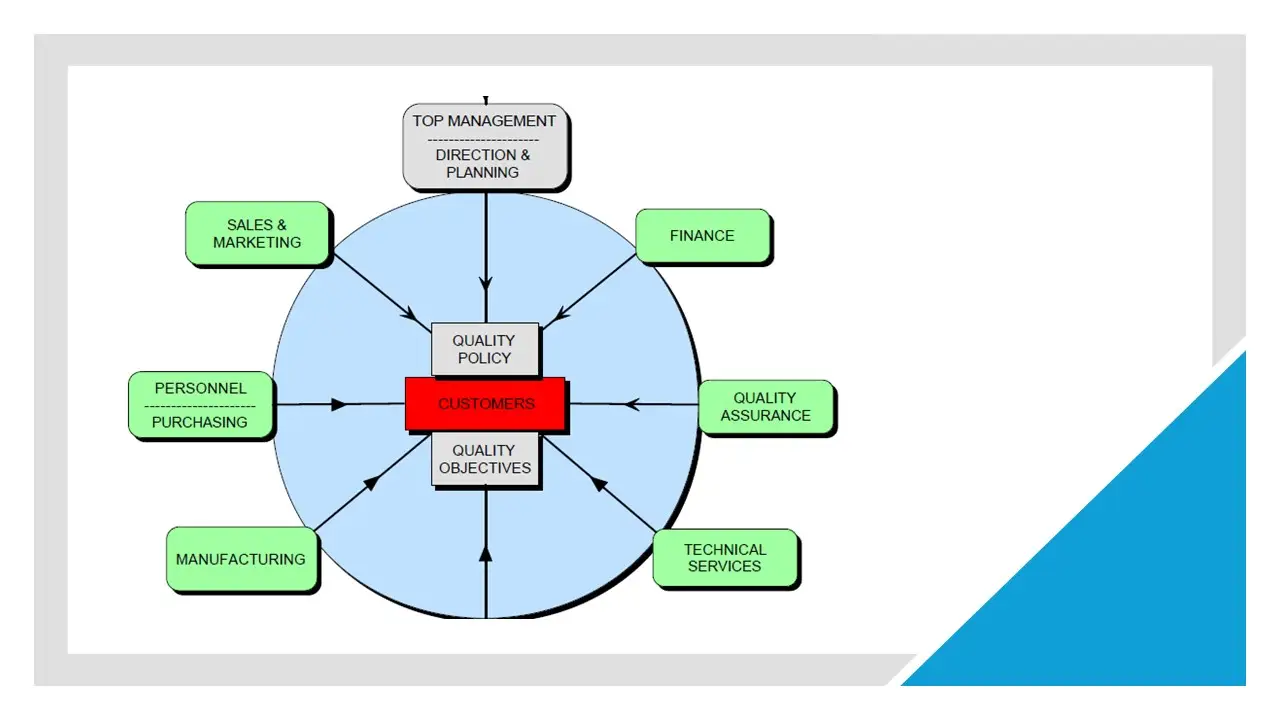
- Operating Principles.Pdf
- Operating Principles.Vsd
- Operatingprinciples.Sdr
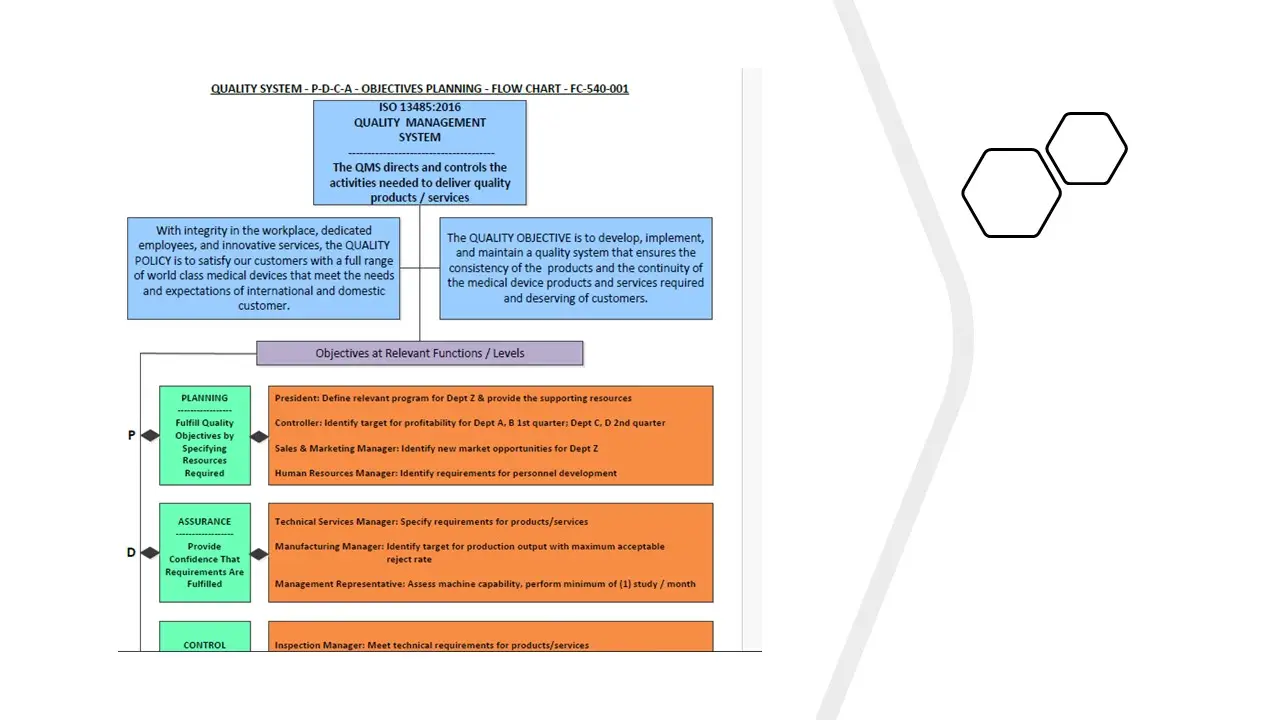
- P-D-C-A Objectives Planning.Pdf
- P-D-C-A Objectives Planning.Sdr
- P-D-C-A Objectives Planning.Vsd
- Organization-Chart.Sdr
- Organization Chart.Pdf
- Organization Chart.Vsd
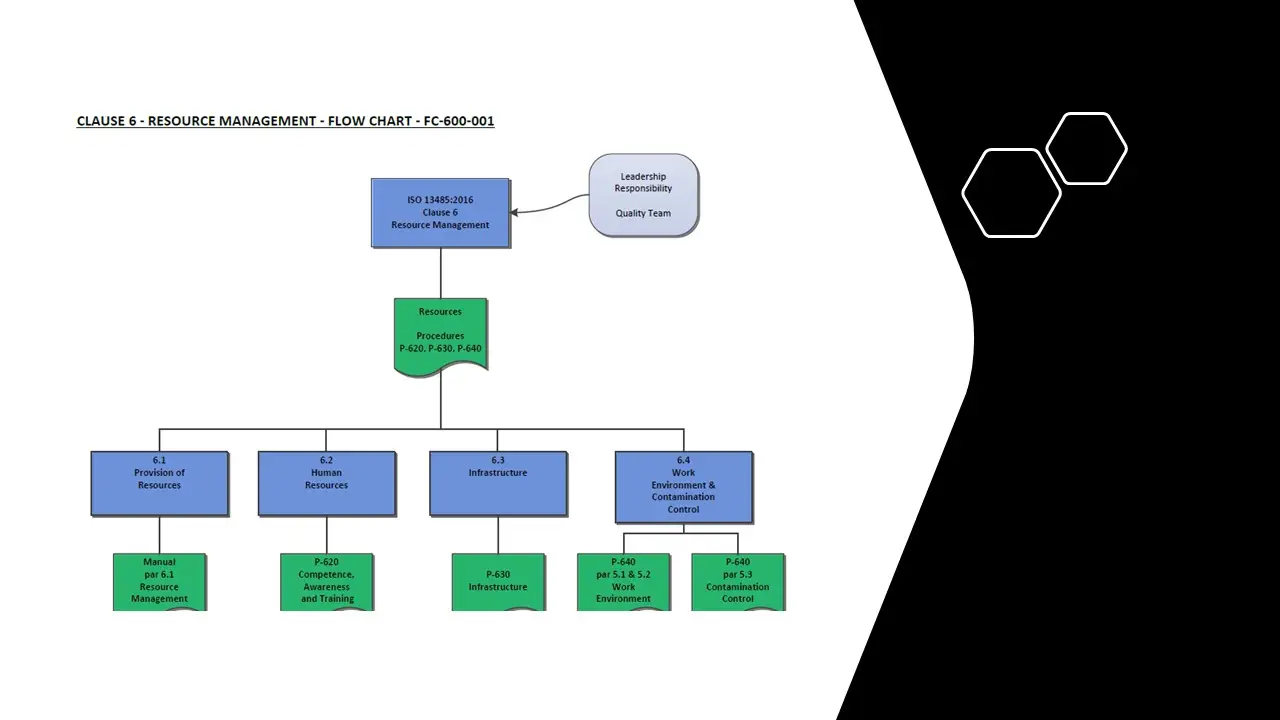
- Clause 6- Resourcemanagement.Sdr
- Clause 6-Resource Management.Pdf
- Clause 6-Resource Management.Vsd
- Contributors-5Ms.Pdf
- Contributors-5Ms.Sdr
- Contributors-5Ms.Vsd
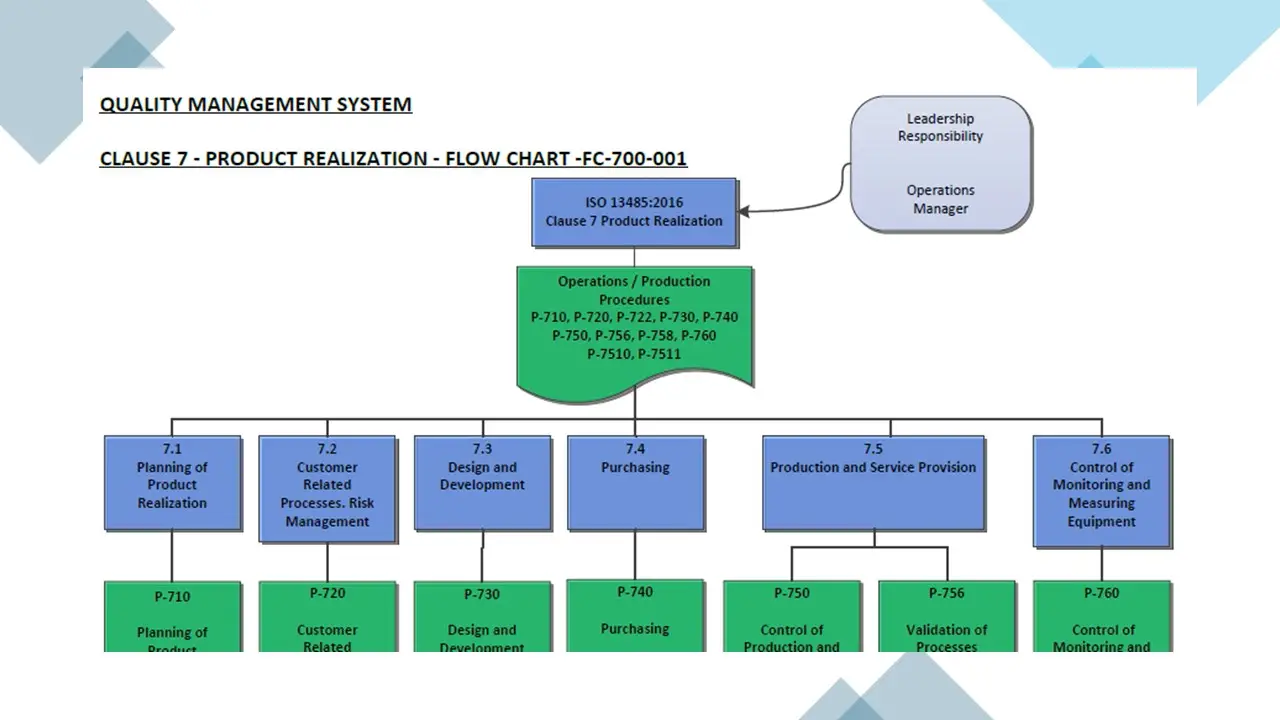
- Clause 7- Product Realization.Pdf
- Clause 7- Product Realization.Vsd
- Clause 7-Productrealization.Sdr
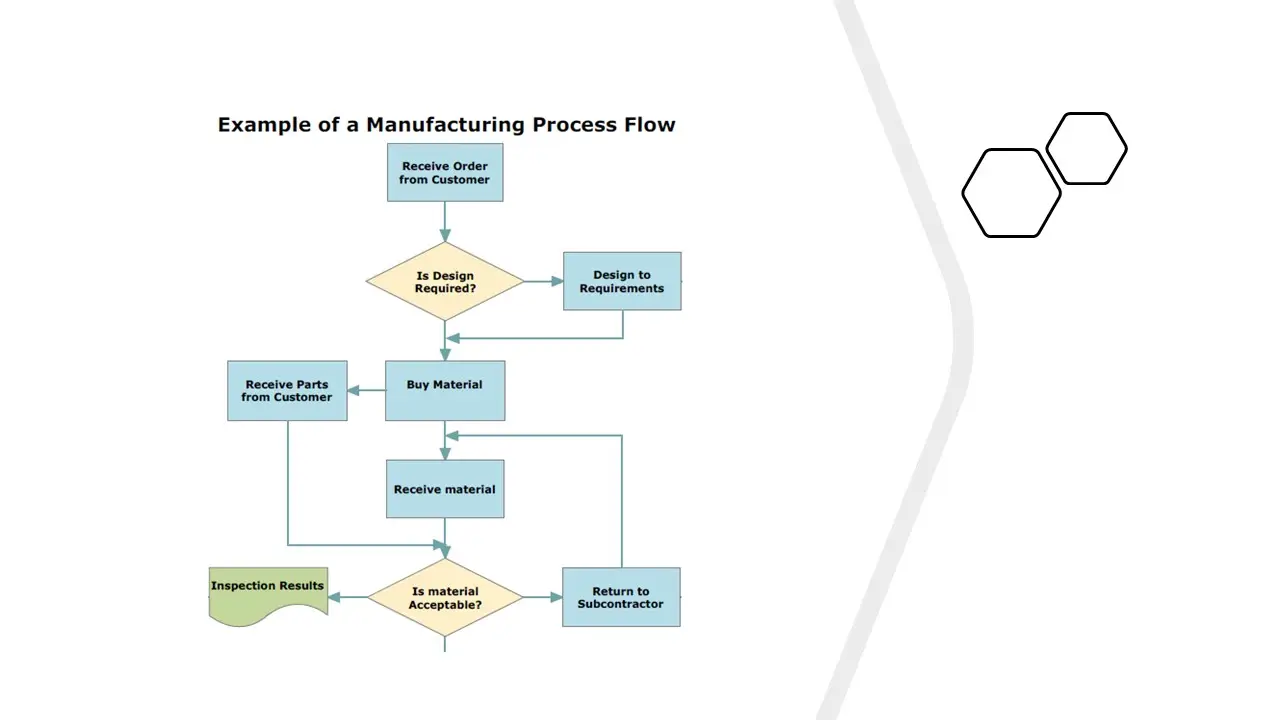
- Process Flow Chart.Pdf
- Process Flow Chart.Vsd
- Processflowchart.Sdr
- Product Realization Quality Plan.Pdf
- Product Realization Quality Plan.Vsd
- Productrealizationqualityplan.Sdr
- Customer Processes.Pdf
- Customer Processes.Vsd
- Customerprocesses.Sdr
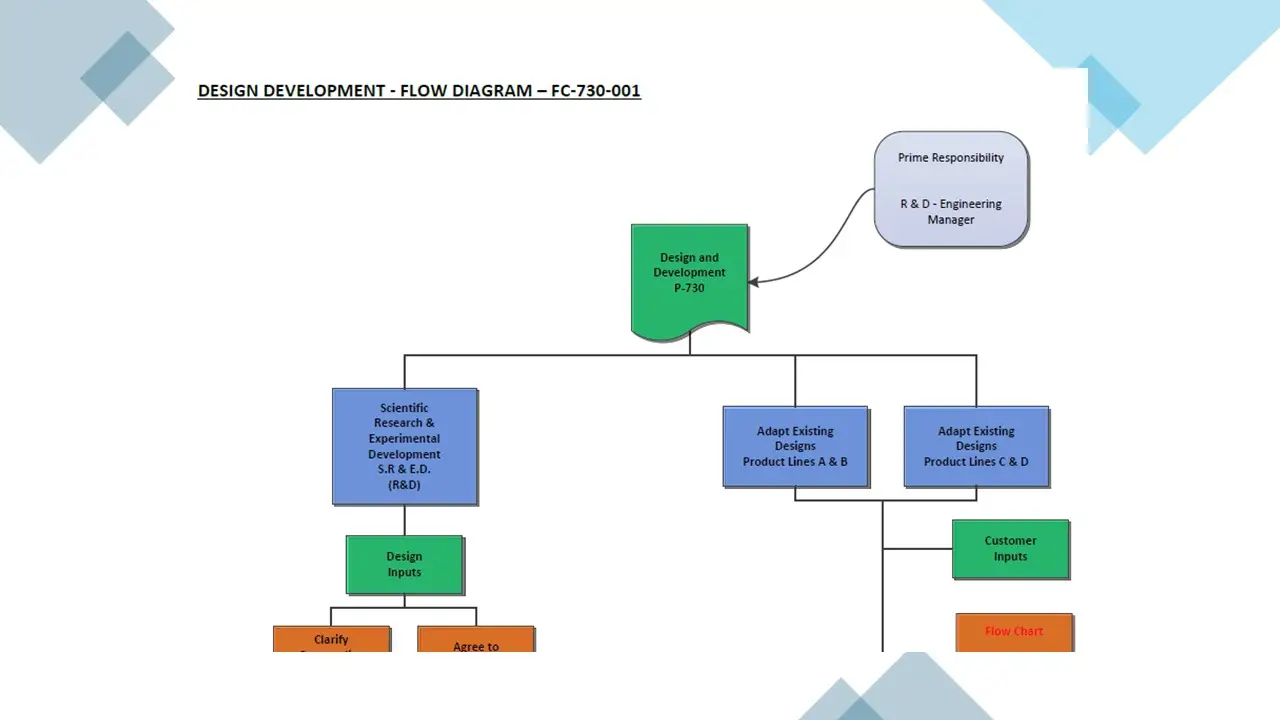
- Design Development.Pdf
- Design Development.Vsd
- Designdevelopment.Sdr
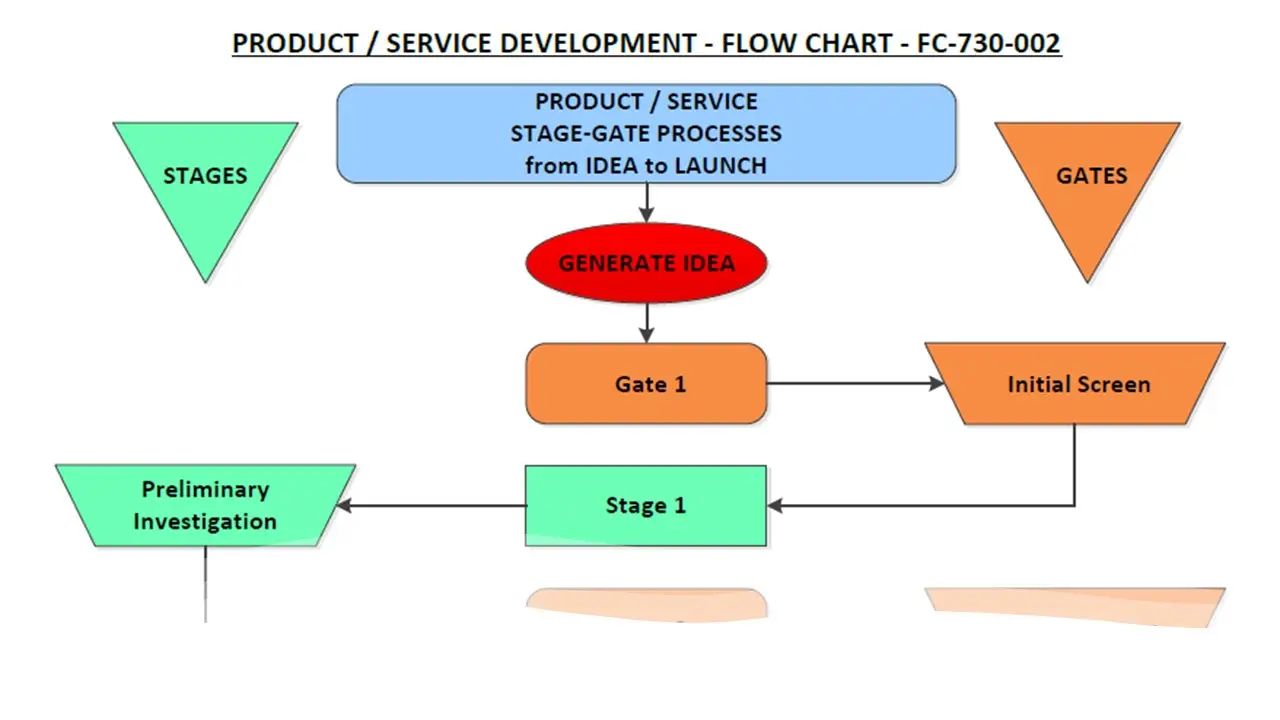
- Stage Gate Idea-To-Launch.Pdf
- Stage Gate Idea-To-Launch.Vsd
- Stagegate Idea-To-Launch.Sdr
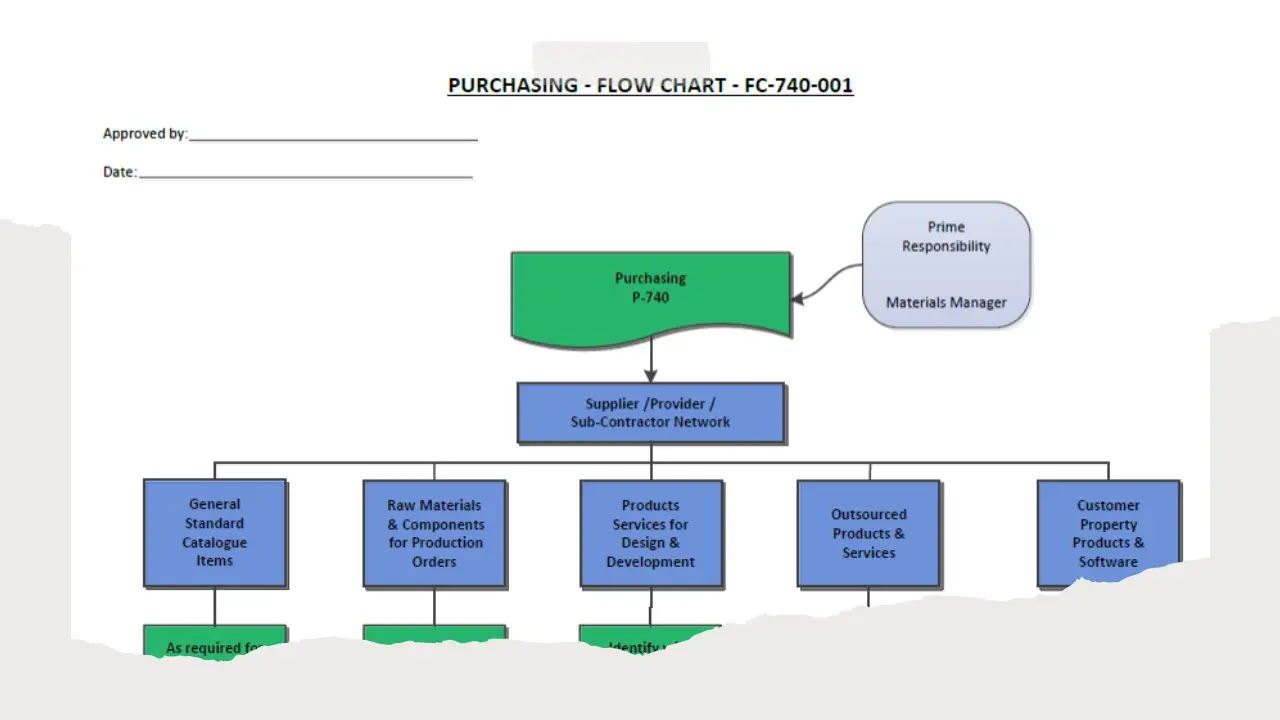
- Purchasing.Pdf
- Purchasing.Sdr
- Purchasing.Vsd
- Process Control.Pdf
- Process Control.Sdr
- Process Control.Vsd
- Process Steps-Control Points.Pdf
- Process Steps-Control Points.Vsd
- Processsteps-Control Points.Sdr
- Identification Traceability.Pdf
- Identification Traceability.Vsd
- Identificationtraceability.Sdr
- Clause 8 – Measure-Improve.Pdf
- Clause 8 – Measure-Improve.Vsd
- Clause 8 -Measure-Improve.Sdr
- Continual Improvement.Pdf
- Continual Improvement.Sdr
- Continual Improvement.Vsd
- Corrective Action.Pdf
- Corrective Action.Sdr
- Corrective Action.Vsd
- 2016 Checklist.Xls
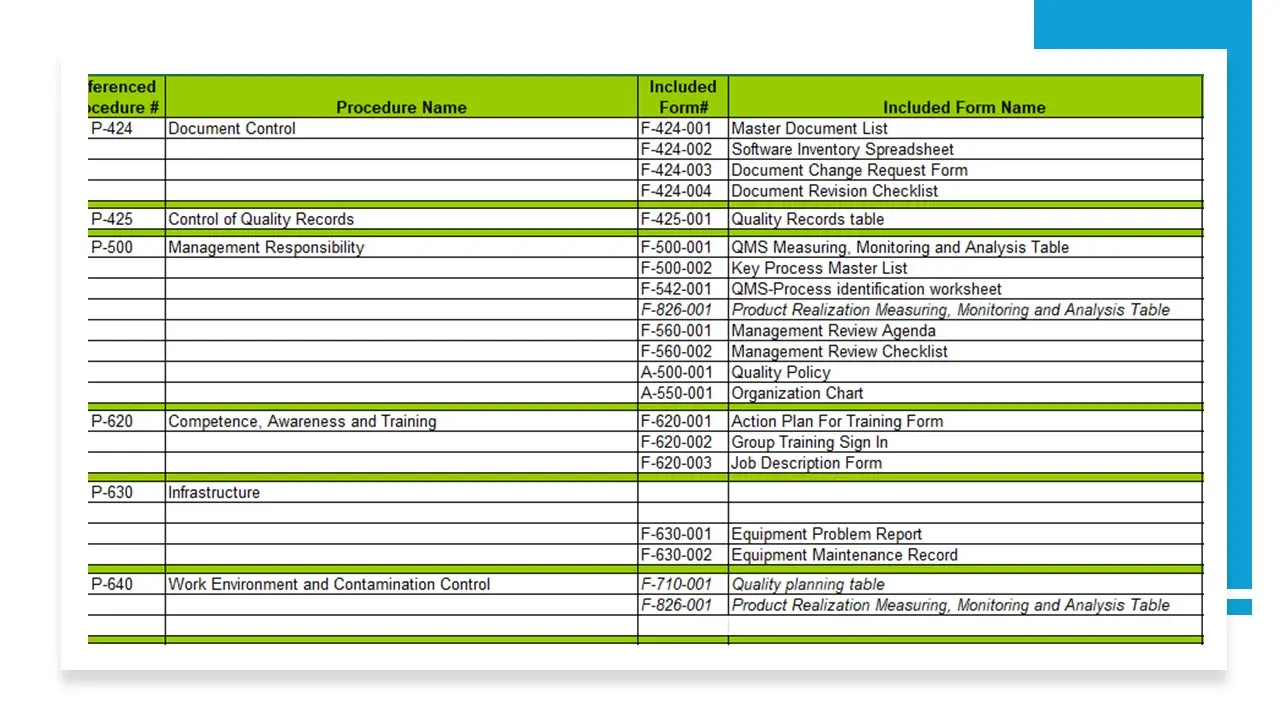
- 2016 Procedures Forms Matrix.Xls
- 2016 Quality Systems Manual.Docx
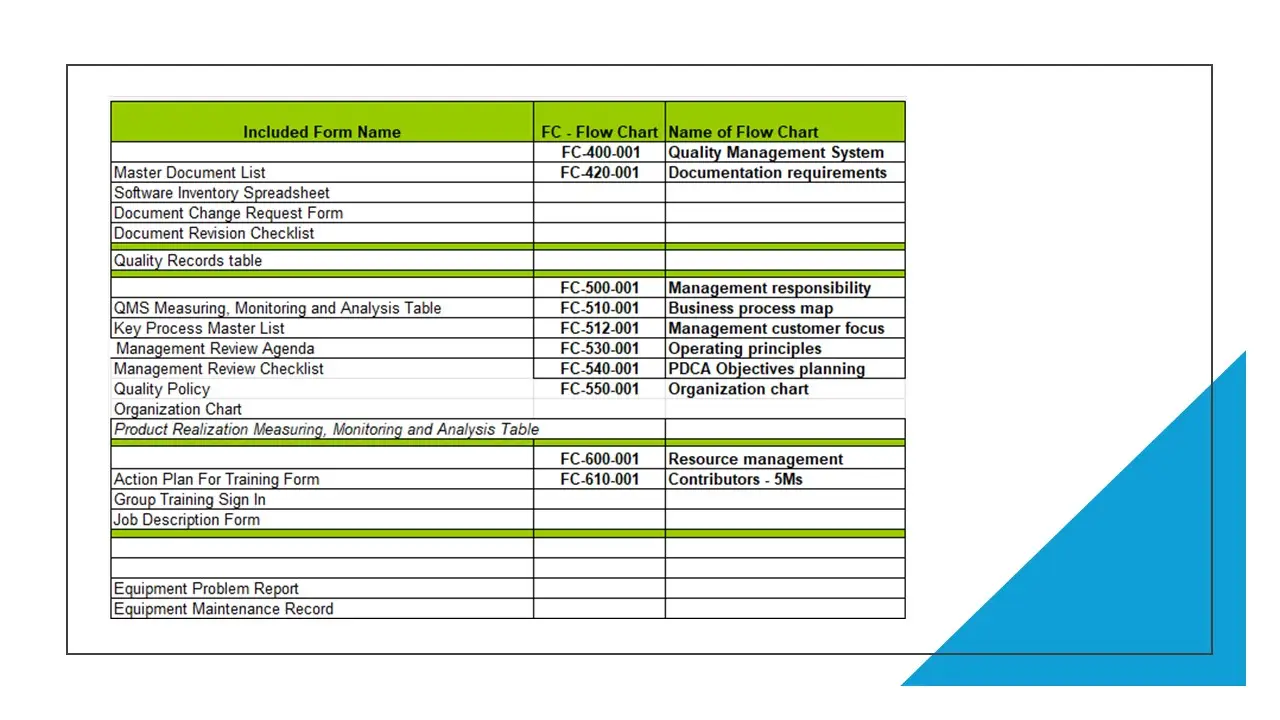
- List And Description Of Quality Management System Flow Charts.Docx
- Document Control.Doc
- Control Of Quality Records.Doc
- Management Responsibility-V2.Doc
- Competence, Awareness And Training.Doc
- Infrastructure.Doc
- Workenvironment-Contaminationcontrol.Doc
- Planning Of Product Realization Processes.Doc
- Customer Related Processes.Doc
- Risk Management.Doc
- Design And Development.Doc
- Purchasing.Doc
- Controlproductionservice-V2.Doc
- Customer Property.Doc
- Preservation Of Product.Doc
- Validation Of Processes For Product Realization.Doc
- Identification And Traceability.Doc
- Control Of Measuring And Monitoring Devices.Doc
- Post Production Feedback.Doc
- Internal Audits.Doc
- Monitoringmeasuringanalysis-Products-Processes.Doc
- Control Of Nonconforming Product.Doc
- Advisorynotices-Productrecall.Doc
- Statistical Techniques.Doc
- Root Cause Analysis.Doc
- Corrective Action.Doc
- Preventive Action.Doc
- Procedures-Forms-Fc-Matrix.Xlsx
- Procedure Template.Doc
- Work Instruction Template.Doc
- Form Template.Doc
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO9001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.







































Reviews
There are no reviews yet.