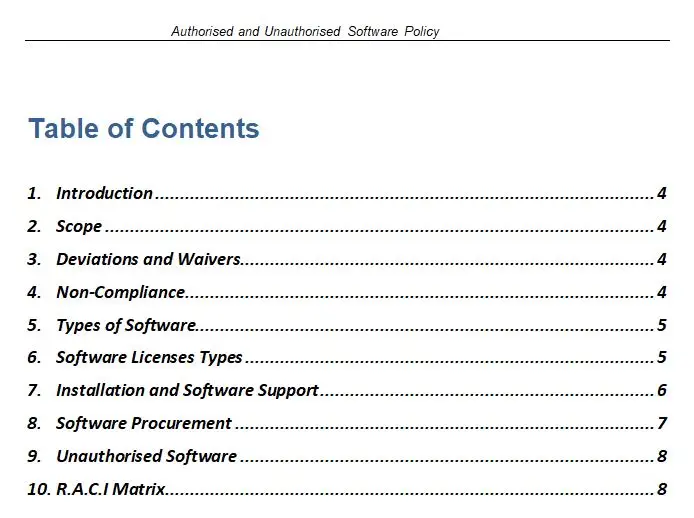
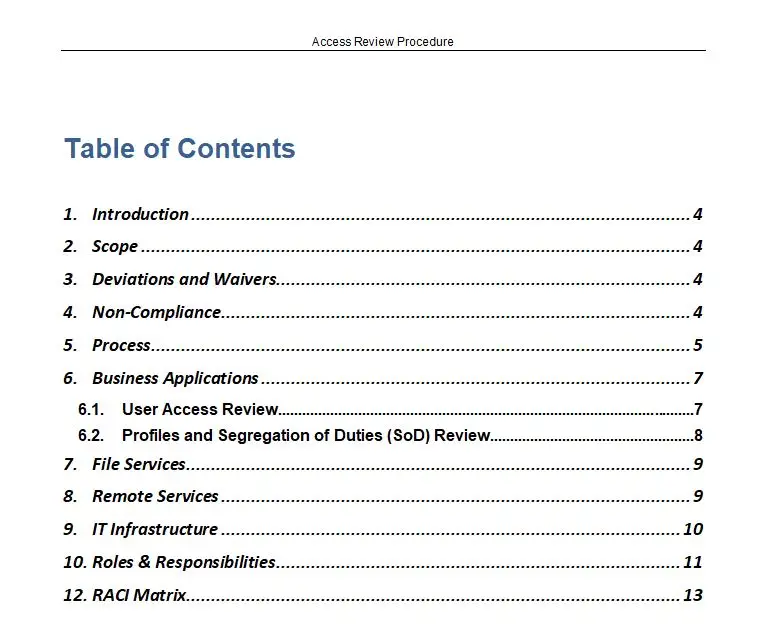
Resource Contributors [5Ms] Flow Chart
In the intricate world of medical device manufacturing, where precision and compliance are paramount, the Resource Contributors [5Ms] Flow Chart emerges as a beacon of clarity and efficiency. This meticulously crafted tool is designed to navigate the complex landscape of ISO 13485, the international standard for quality management systems in the medical device industry. The flowchart serves as an indispensable guide for organizations striving to meet the rigorous demands of this standard, ensuring that every resource contributor is aligned with the overarching goals of quality and safety.
At the heart of this flowchart lies the concept of the 5Ms: Manpower, Methods, Machines, Materials, and Measurement. These five pillars are the foundation upon which successful medical device manufacturing is built. The Resource Contributors [5Ms] Flow Chart provides a comprehensive visual representation of how these elements interact and contribute to the overall quality management system. Available in versatile formats such as PDF, SDR, and Visio, this flowchart is accessible to a wide range of users, from quality managers to production engineers, offering a seamless integration into existing workflows.
One of the key features of the Resource Contributors [5Ms] Flow Chart is its ability to simplify complex processes. By breaking down the intricate web of resource contributions into a clear and concise visual format, it empowers organizations to identify potential bottlenecks and areas for improvement. This not only enhances operational efficiency but also ensures compliance with ISO 13485, reducing the risk of costly non-conformities and product recalls.
The flowchart’s value proposition extends beyond mere compliance. It serves as a strategic tool for continuous improvement, fostering a culture of quality and accountability within the organization. By providing a holistic view of resource contributions, it enables stakeholders to make informed decisions, optimize resource allocation, and ultimately deliver safer and more effective medical devices to the market.
In the competitive landscape of medical device manufacturing, the Resource Contributors [5Ms] Flow Chart stands out as a vital asset. It bridges the gap between regulatory requirements and practical implementation, offering a roadmap to success in the pursuit of excellence. As part of the broader category of All Products and ISO 13485, this flowchart is a testament to the commitment to quality and innovation that defines the industry.
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.

![ISO 13485 ISO 13485 Optimize your medical device manufacturing process with the Resource Contributors [5Ms] Flow Chart, a vital tool for ISO 13485 compliance. This comprehensive flowchart, available in PDF, SDR, and Visio formats, visually represents the 5Ms—Manpower, Methods, Machines, Materials, and Measurement—ensuring quality and safety in your operations. Ideal for quality managers and production engineers, it simplifies complex processes, enhances efficiency, and supports continuous improvement. Elevate your quality management system with this indispensable resource.](https://governancedocs.com/wp-content/uploads/2024/10/ISO-13485.webp)
![Resource Contributors [5Ms] Flow Chart 1 ISO 13485 Optimize your medical device manufacturing process with the Resource Contributors [5Ms] Flow Chart, a vital tool for ISO 13485 compliance. This comprehensive flowchart, available in PDF, SDR, and Visio formats, visually represents the 5Ms—Manpower, Methods, Machines, Materials, and Measurement—ensuring quality and safety in your operations. Ideal for quality managers and production engineers, it simplifies complex processes, enhances efficiency, and supports continuous improvement. Elevate your quality management system with this indispensable resource.](https://governancedocs.com/wp-content/uploads/2024/10/ISO-13485-100x100.webp)


![Access Review Procedure Template 4 02 3D Mockup Optimize your medical device manufacturing process with the Resource Contributors [5Ms] Flow Chart, a vital tool for ISO 13485 compliance. This comprehensive flowchart, available in PDF, SDR, and Visio formats, visually represents the 5Ms—Manpower, Methods, Machines, Materials, and Measurement—ensuring quality and safety in your operations. Ideal for quality managers and production engineers, it simplifies complex processes, enhances efficiency, and supports continuous improvement. Elevate your quality management system with this indispensable resource.](https://governancedocs.com/wp-content/uploads/2023/11/02-3D-Mockup.webp)

Reviews
There are no reviews yet