Procedure for Non-Conformity Reporting and Corrective Action
In the intricate world of quality management, where precision and compliance are paramount, the Procedure for Non-Conformity Reporting and Corrective Action emerges as a beacon of structured excellence. This product, identified by the number 8022, is not just a tool but a transformative process that aligns seamlessly with the rigorous standards of ISO 9001, ensuring that organizations not only meet but exceed quality expectations.
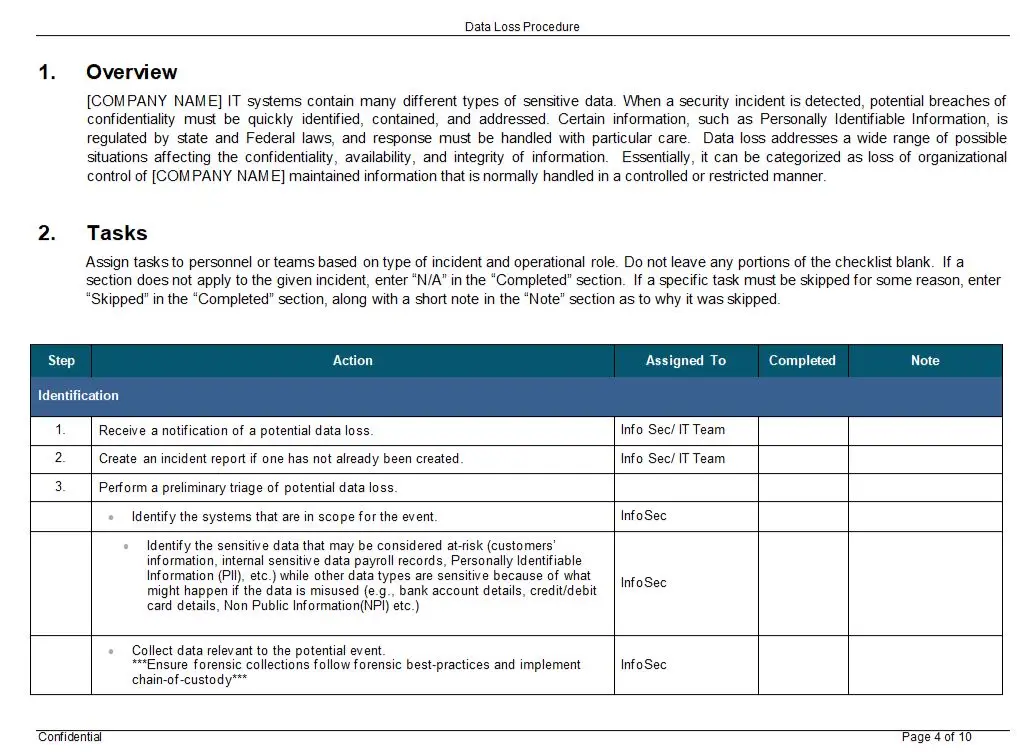
At its core, this procedure is a meticulously crafted framework designed to identify, report, and rectify non-conformities within an organization. It serves as a critical component in the quality management system, providing a clear and systematic approach to addressing deviations from established standards. The procedure is not merely about compliance; it is about fostering a culture of continuous improvement and operational excellence.
Key features of this procedure include a comprehensive reporting mechanism that captures non-conformities with precision and clarity. It empowers employees at all levels to report issues without fear of retribution, thereby promoting transparency and accountability. The procedure also outlines a robust corrective action plan, which is essential for addressing the root causes of non-conformities and preventing their recurrence. This plan is not a one-size-fits-all solution but is tailored to the specific needs and challenges of each organization, ensuring that corrective actions are both effective and sustainable.
The benefits of implementing this procedure are manifold. Organizations that adopt this structured approach to non-conformity reporting and corrective action can expect to see significant improvements in their quality management processes. By systematically addressing non-conformities, they can reduce waste, enhance product quality, and increase customer satisfaction. Moreover, compliance with ISO 9001 standards not only boosts an organization’s reputation but also opens doors to new business opportunities and markets.
The value proposition of the Procedure for Non-Conformity Reporting and Corrective Action lies in its ability to transform potential setbacks into opportunities for growth and improvement. It equips organizations with the tools they need to navigate the complexities of quality management with confidence and agility. By embedding this procedure into their operations, organizations can build a resilient quality management system that stands the test of time.
In the realm of All Products and ISO 9001, this procedure stands out as a vital asset for any organization committed to achieving and maintaining the highest standards of quality. It is more than just a procedure; it is a strategic investment in the future of the organization, ensuring that it remains competitive and compliant in an ever-evolving business landscape.
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.







Reviews
There are no reviews yet