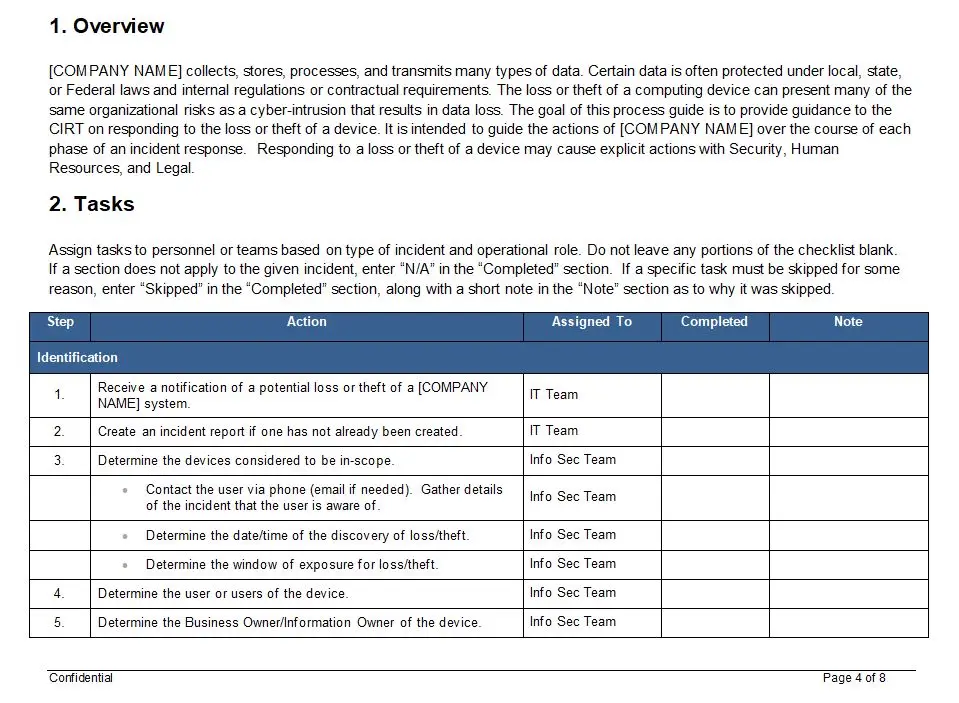
Clause 7 – Product Realization Flow Chart
In the intricate world of medical device manufacturing, where precision and compliance are paramount, the Clause 7 – Product Realization Flow Chart emerges as a beacon of clarity and efficiency. This indispensable tool is meticulously designed to guide organizations through the labyrinthine process of product realization, ensuring adherence to the stringent standards of ISO 13485.
At its core, the Clause 7 – Product Realization Flow Chart is a comprehensive visual representation that delineates each step of the product realization process. Available in versatile formats such as PDF, SDR, and Visio, it caters to diverse organizational needs, providing flexibility and ease of integration into existing systems. The flowchart is not merely a static document; it is a dynamic roadmap that transforms complex regulatory requirements into actionable insights, empowering teams to navigate the product development lifecycle with confidence and precision.
Key features of this flowchart include its intuitive design and detailed breakdown of processes, which collectively demystify the often-daunting task of compliance. Each phase of product realization, from initial concept to final delivery, is meticulously charted, ensuring that no critical step is overlooked. This level of detail is crucial for organizations striving to meet the rigorous demands of ISO 13485, a standard that underscores the importance of quality management systems in the medical device industry.
The benefits of utilizing the Clause 7 – Product Realization Flow Chart are manifold. By providing a clear and concise framework, it reduces the risk of non-compliance, which can lead to costly delays and potential market setbacks. Moreover, it fosters a culture of quality and continuous improvement, as teams are equipped with a tool that not only guides but also educates. The flowchart serves as a training aid, enhancing the understanding of ISO 13485 requirements across all levels of an organization, from new hires to seasoned professionals.
The value proposition of the Clause 7 – Product Realization Flow Chart lies in its ability to streamline operations and enhance productivity. By offering a standardized approach to product realization, it minimizes variability and ensures consistency in output. This is particularly valuable in the medical device sector, where even minor deviations can have significant implications. Furthermore, the flowchart supports strategic decision-making by providing a clear overview of the entire process, enabling organizations to identify bottlenecks and optimize resource allocation.
In conclusion, the Clause 7 – Product Realization Flow Chart is more than just a tool; it is an essential component of a robust quality management system. Its comprehensive nature and user-friendly design make it an invaluable asset for any organization committed to excellence in the medical device industry. By aligning processes with ISO 13485 standards, it not only facilitates compliance but also drives innovation and growth, ensuring that products are realized efficiently and effectively, from concept to market.
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.







Reviews
There are no reviews yet