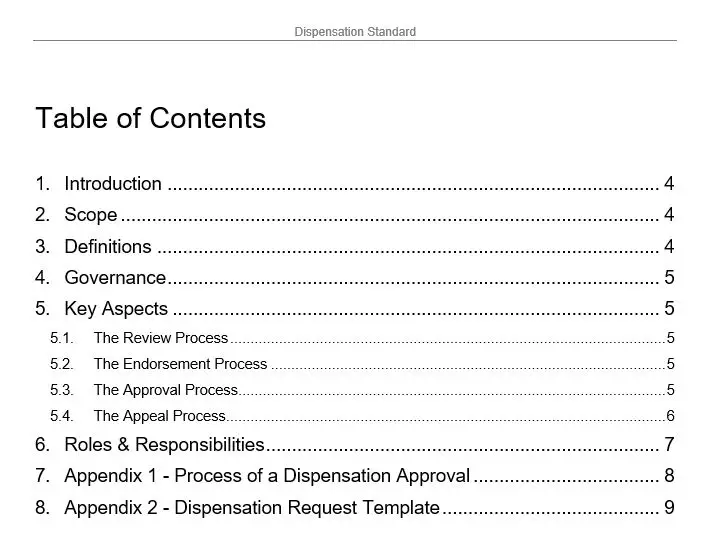
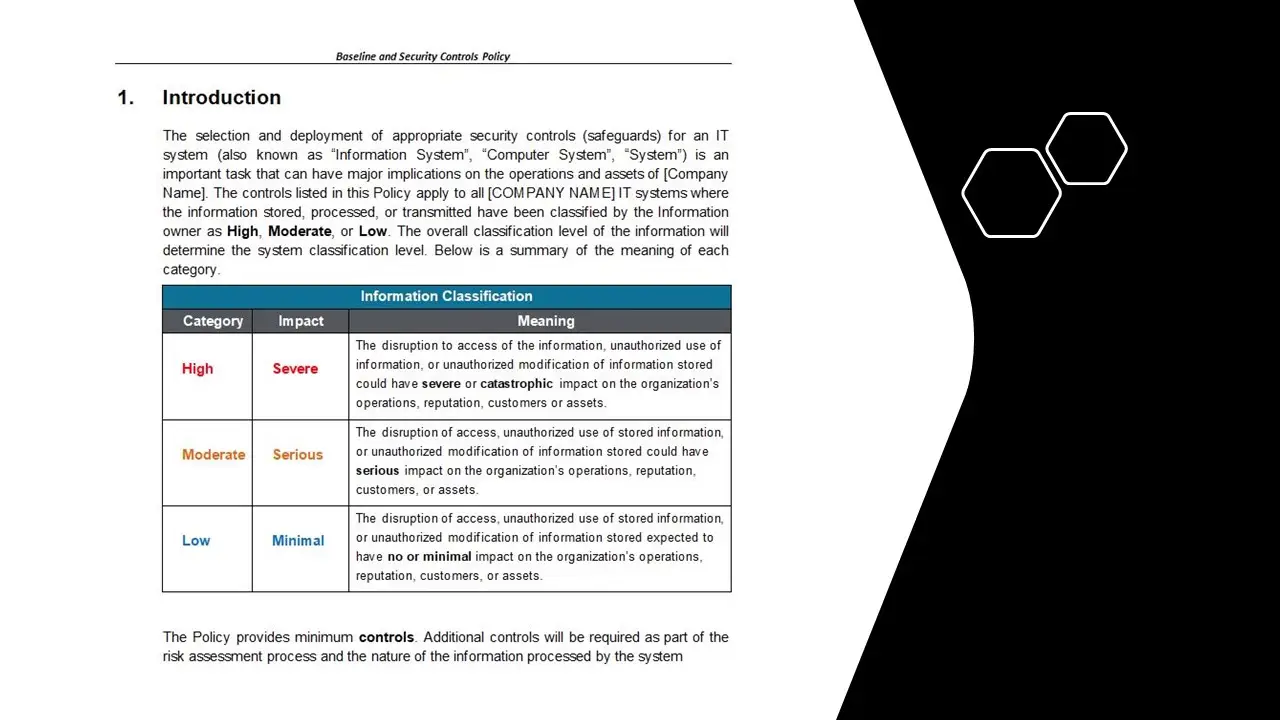
Quality Records Table
In the intricate world of medical device manufacturing, where precision and compliance are paramount, the Quality Records Table emerges as an indispensable tool for organizations striving to meet the rigorous standards of ISO 13485. This table template is not just a mere document; it is a meticulously crafted solution designed to streamline the management and organization of quality records, ensuring that every piece of data is meticulously cataloged and easily accessible.
At the heart of the Quality Records Table is its unique ability to transform the daunting task of quality management into a seamless and efficient process. With the product number 8022, this template stands as a beacon of reliability and excellence, offering a structured framework that aligns perfectly with the stringent requirements of ISO 13485. It is a testament to the commitment of organizations to uphold the highest standards of quality and safety in the production of medical devices.
The key features of the Quality Records Table are tailored to meet the diverse needs of quality management professionals. It provides a comprehensive layout that allows for the systematic recording of quality data, ensuring that every record is meticulously documented and easily retrievable. The template is designed to accommodate a wide range of quality records, from design and development documentation to production and post-market surveillance data. This versatility makes it an invaluable asset for organizations seeking to maintain a robust quality management system.
One of the standout benefits of the Quality Records Table is its ability to enhance organizational efficiency. By providing a centralized repository for quality records, it eliminates the chaos of scattered documentation and reduces the risk of non-compliance. This not only saves time but also ensures that organizations are always audit-ready, with all necessary documentation at their fingertips. The template’s intuitive design allows for easy customization, enabling organizations to tailor it to their specific needs and processes.
The value proposition of the Quality Records Table lies in its ability to bridge the gap between compliance and operational excellence. It empowers organizations to not only meet regulatory requirements but also to exceed them, fostering a culture of continuous improvement and quality assurance. By integrating this template into their quality management systems, organizations can enhance their reputation for reliability and trustworthiness, gaining a competitive edge in the market.
In the realm of All Products and ISO 13485, the Quality Records Table stands out as a vital tool for any organization committed to quality and compliance. It is more than just a template; it is a strategic asset that drives efficiency, ensures compliance, and supports the overarching goal of delivering safe and effective medical devices to the market. With the Quality Records Table, organizations can confidently navigate the complexities of quality management, knowing that they have a reliable partner in their pursuit of excellence.
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.








Reviews
There are no reviews yet