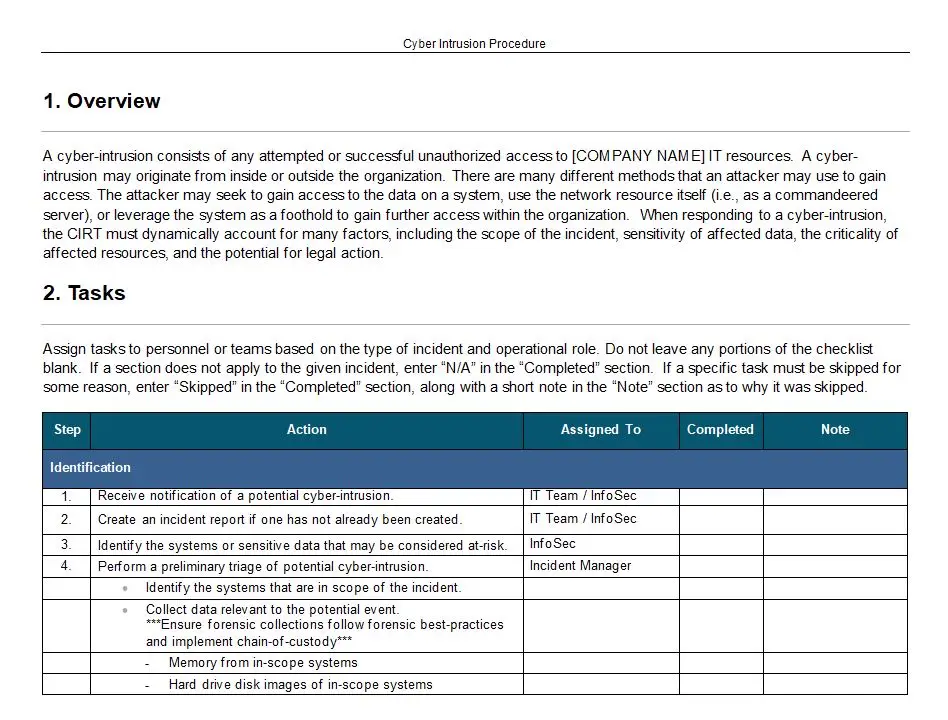
Product Realization Measuring Monitoring And Analysis Table
In the intricate world of product development, where precision and compliance are paramount, the “Product Realization Measuring Monitoring And Analysis Table” emerges as an indispensable tool. This table template, identified by the number 8022, is meticulously designed to streamline the measurement and analysis of product realization processes, ensuring that every step from conception to market release is executed with unparalleled accuracy and efficiency.
At the heart of this table’s design is its alignment with ISO 13485 standards, a globally recognized benchmark for quality management systems in the medical device industry. This alignment ensures that the table not only meets but exceeds the rigorous demands of regulatory compliance, making it an essential asset for organizations striving to maintain the highest quality standards.
The table’s key features are a testament to its comprehensive nature. It provides a structured framework that allows for the systematic tracking of product development stages, from initial design inputs to final product verification and validation. Each section of the table is crafted to capture critical data points, facilitating a thorough analysis of the processes involved. This level of detail ensures that potential issues are identified early, allowing for timely interventions that can prevent costly delays or non-compliance issues.
One of the standout benefits of the Product Realization Measuring Monitoring And Analysis Table is its ability to enhance communication and collaboration across different departments. By providing a clear and concise overview of the product realization process, it enables teams to work in harmony, ensuring that everyone is aligned with the project’s objectives and timelines. This collaborative approach not only boosts efficiency but also fosters a culture of continuous improvement, where feedback is actively sought and integrated into future projects.
Moreover, the table’s user-friendly design makes it accessible to all stakeholders, regardless of their technical expertise. Its intuitive layout and clear instructions mean that it can be easily adopted by teams, reducing the learning curve and allowing organizations to quickly reap the benefits of its implementation.
In terms of value proposition, the Product Realization Measuring Monitoring And Analysis Table offers a unique blend of compliance, efficiency, and quality assurance. By integrating this tool into their processes, organizations can significantly reduce the risk of non-compliance, enhance product quality, and accelerate time-to-market. This not only leads to cost savings but also strengthens the organization’s reputation as a leader in quality and innovation.
In conclusion, the Product Realization Measuring Monitoring And Analysis Table is more than just a template; it is a strategic asset that empowers organizations to achieve excellence in product development. Its comprehensive features, coupled with its alignment with ISO 13485 standards, make it an essential tool for any organization committed to delivering high-quality products in a competitive market.
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.








Reviews
There are no reviews yet