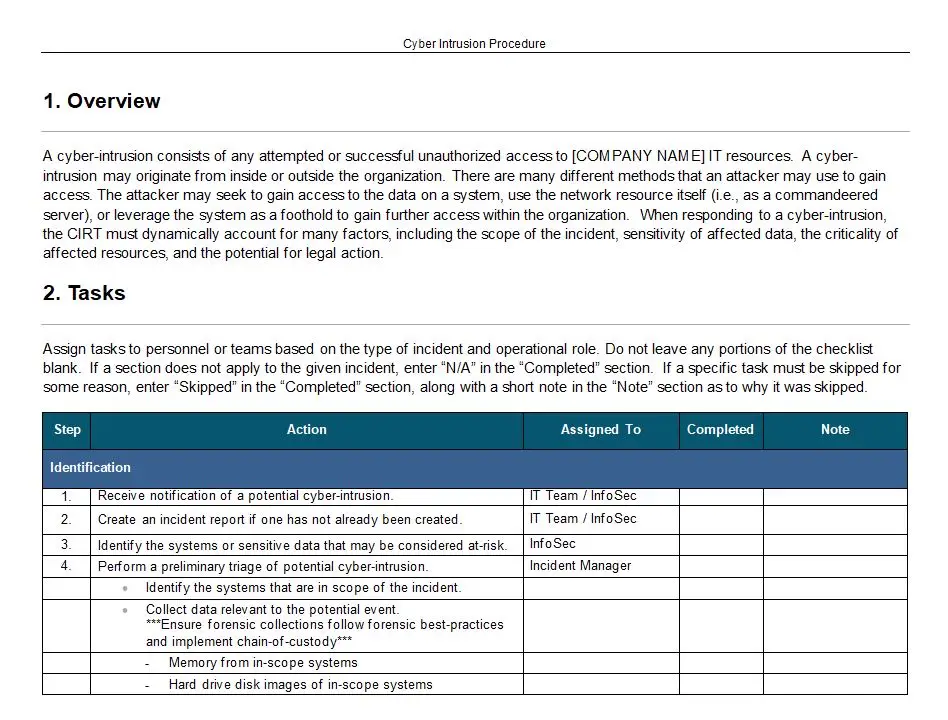
Document Control Procedures
In the intricate world of quality management systems, where precision and compliance are paramount, Document Control Procedures emerge as the unsung hero, orchestrating the symphony of order and efficiency. Imagine a bustling manufacturing facility, where every cog in the machine must align perfectly to produce excellence. Here, Document Control Procedures, identified by the code 8022, serve as the backbone, ensuring that every document, from the initial design blueprint to the final quality assurance report, is meticulously managed and controlled.
At its core, Document Control Procedures are the guardians of a quality system, particularly within the rigorous framework of ISO 13485. This standard, revered in the medical device industry, demands an unwavering commitment to quality and safety. Document Control Procedures rise to this challenge, offering a robust set of guidelines that govern the creation, review, approval, distribution, and archiving of documents. These procedures are not mere bureaucratic hurdles; they are the lifeline that ensures every document is current, accurate, and accessible to those who need it.
The key features of Document Control Procedures are as diverse as they are essential. They provide a structured approach to document management, incorporating version control to prevent the chaos of outdated information. With a keen eye on compliance, these procedures ensure that every document undergoes rigorous review and approval processes, safeguarding against errors that could compromise quality. Furthermore, they establish a clear hierarchy of access, ensuring that sensitive information is only available to authorized personnel.
The benefits of implementing Document Control Procedures are manifold. For organizations striving for excellence, these procedures offer a pathway to enhanced efficiency and reduced risk. By maintaining a centralized repository of documents, they eliminate the time-consuming search for information, allowing employees to focus on their core tasks. Moreover, in an era where regulatory scrutiny is ever-increasing, Document Control Procedures provide a shield of compliance, reducing the risk of costly fines and reputational damage.
The value proposition of Document Control Procedures is compelling. They are not just a set of rules; they are a strategic asset that empowers organizations to achieve their quality objectives. By fostering a culture of accountability and transparency, these procedures enhance collaboration and communication across departments. They transform document management from a mundane task into a strategic advantage, enabling organizations to respond swiftly to market demands and regulatory changes.
In the grand tapestry of All Products, Document Control Procedures stand out as a beacon of reliability and excellence. They are the silent architects of quality, ensuring that every document is a testament to an organization’s commitment to precision and compliance. As industries evolve and standards become more stringent, the role of Document Control Procedures will only grow in significance, guiding organizations toward a future where quality is not just a goal but a way of life.
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.








Reviews
There are no reviews yet