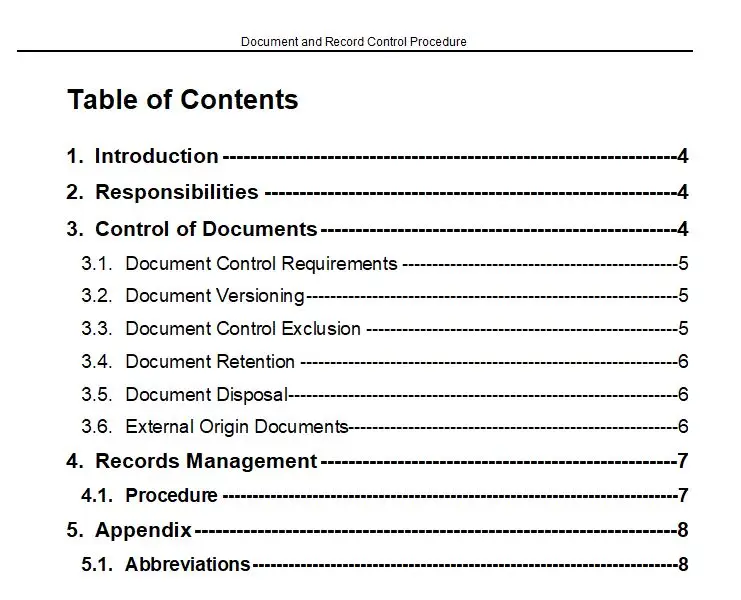
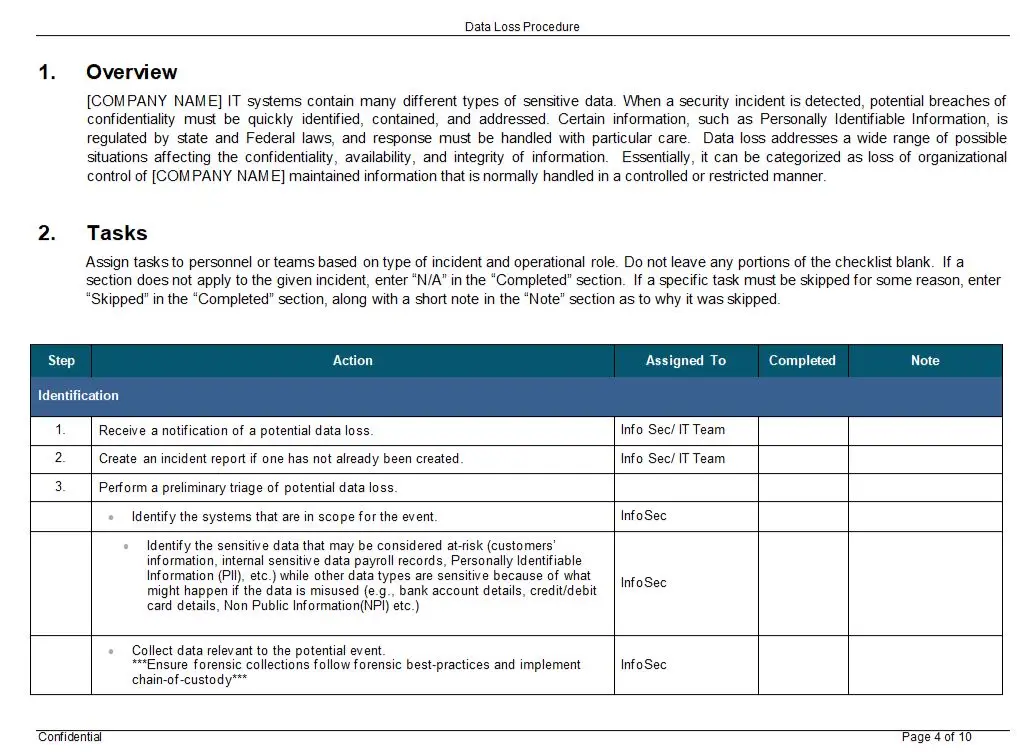
Clause 5 – Management Responsibility Flow Chart
In the intricate world of quality management systems, where precision and clarity are paramount, the Clause 5 – Management Responsibility Flow Chart emerges as a beacon of guidance and efficiency. This meticulously crafted tool is not just a flowchart; it is a comprehensive roadmap designed to illuminate the path of management responsibilities within the framework of ISO 13485, the international standard for medical device quality management systems.
At its core, the Clause 5 – Management Responsibility Flow Chart is a visual masterpiece, available in versatile formats such as PDF, SDR, and Visio. This flexibility ensures that it seamlessly integrates into any organization’s existing documentation and workflow processes. Whether you are a seasoned quality manager or a newcomer to the field, this flowchart is designed to be intuitive and accessible, breaking down complex management responsibilities into clear, actionable steps.
One of the key features of this flowchart is its ability to distill the essence of ISO 13485’s Clause 5 into a visual format that is both comprehensive and easy to understand. It captures the essence of management commitment, customer focus, quality policy, planning, responsibility, authority, communication, and management review. Each element is meticulously detailed, ensuring that no aspect of management responsibility is overlooked.
The benefits of utilizing the Clause 5 – Management Responsibility Flow Chart are manifold. For organizations striving for ISO 13485 certification, it serves as an invaluable tool to ensure compliance with the standard’s stringent requirements. By providing a clear depiction of management responsibilities, it aids in aligning organizational processes with regulatory expectations, thereby reducing the risk of non-compliance and enhancing overall operational efficiency.
Moreover, the flowchart acts as a catalyst for fostering a culture of quality within the organization. By clearly delineating roles and responsibilities, it empowers employees at all levels to understand their contributions to the quality management system. This not only enhances accountability but also drives continuous improvement, as teams are better equipped to identify and address potential issues before they escalate.
The value proposition of the Clause 5 – Management Responsibility Flow Chart extends beyond compliance and operational efficiency. It is a strategic tool that enables organizations to build trust with stakeholders, including customers, regulatory bodies, and partners. By demonstrating a commitment to quality and regulatory compliance, organizations can enhance their reputation and gain a competitive edge in the market.
In conclusion, the Clause 5 – Management Responsibility Flow Chart is more than just a product; it is a strategic asset for any organization operating within the medical device industry. Its comprehensive depiction of management responsibilities, coupled with its ease of use and integration, makes it an indispensable tool for achieving ISO 13485 compliance and driving organizational excellence. As part of the broader category of All Products under ISO 13485, it stands out as a testament to the power of visual tools in enhancing quality management systems.
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.








Reviews
There are no reviews yet