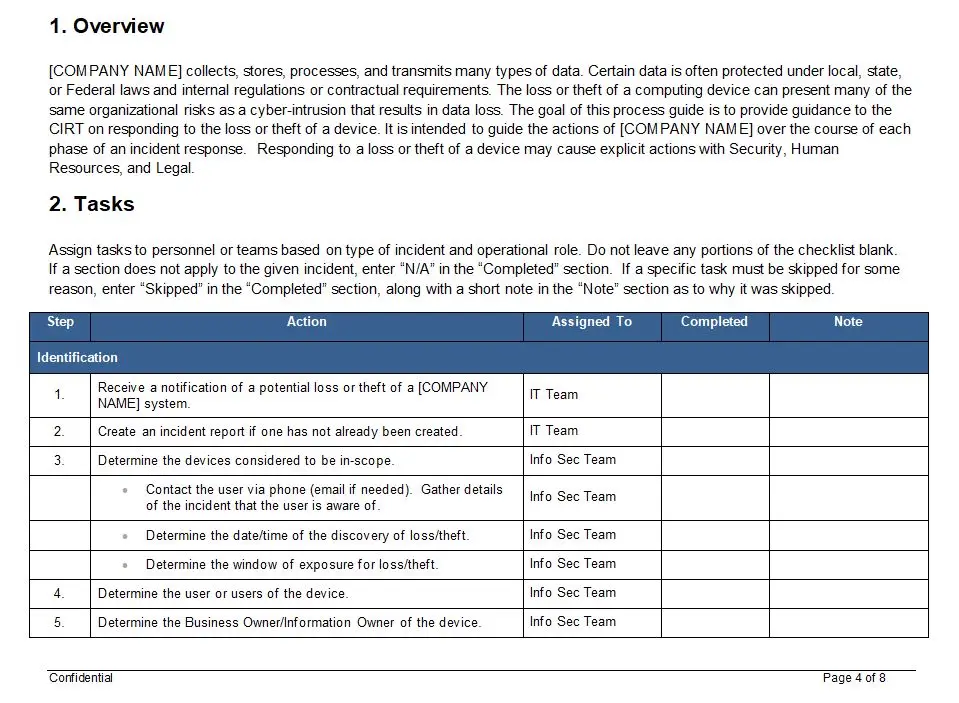
Action Plan For Training Form
In the fast-paced world of medical device manufacturing, where precision and compliance are paramount, the “Action Plan For Training Form” emerges as an indispensable tool, guiding organizations through the intricate maze of training requirements. This form, identified by the unique number 8022, is not just a document; it is a strategic blueprint designed to ensure that every step and action necessary for comprehensive medical device training is meticulously outlined and executed.
Imagine a bustling medical device company, where innovation meets regulation. Here, the stakes are high, and the margin for error is non-existent. The “Action Plan For Training Form” becomes the cornerstone of the training process, ensuring that every team member is equipped with the knowledge and skills required to maintain the highest standards of quality and safety. This form is a testament to the company’s commitment to excellence, aligning with the rigorous demands of ISO 13485, the international standard for quality management systems in the medical device industry.
The key features of this form are its structured approach and comprehensive coverage. It provides a clear framework for identifying training needs, setting objectives, and detailing the specific actions required to achieve those objectives. Each section of the form is meticulously crafted to capture essential information, from the identification of training gaps to the assignment of responsibilities and timelines. This ensures that nothing is left to chance, and every aspect of the training process is accounted for.
The benefits of using the “Action Plan For Training Form” are manifold. For one, it enhances organizational efficiency by streamlining the training process, reducing redundancy, and ensuring that resources are allocated effectively. It also fosters a culture of continuous improvement, as it encourages regular review and updating of training plans to reflect the latest industry standards and technological advancements. Moreover, by providing a clear and documented training plan, it helps organizations demonstrate compliance with ISO 13485, thereby enhancing their credibility and reputation in the market.
The value proposition of this form lies in its ability to transform the training process from a mundane task into a strategic initiative. It empowers organizations to take a proactive approach to training, anticipating challenges and addressing them before they become issues. This not only improves the quality of the training but also boosts employee morale and engagement, as team members feel more confident and competent in their roles.
In conclusion, the “Action Plan For Training Form” is more than just a document; it is a catalyst for change, driving organizations towards greater efficiency, compliance, and excellence. It is a vital component of any medical device company’s toolkit, ensuring that they remain at the forefront of innovation while adhering to the highest standards of quality and safety. As the industry continues to evolve, this form will remain a steadfast ally, guiding organizations through the complexities of training with precision and foresight.
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.








Reviews
There are no reviews yet