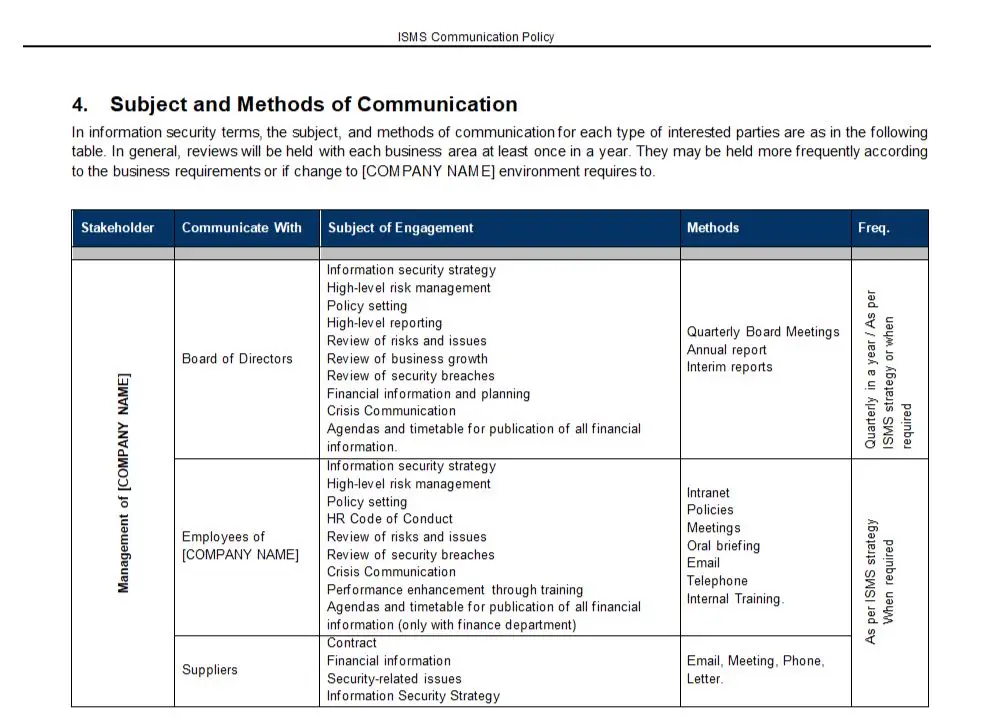
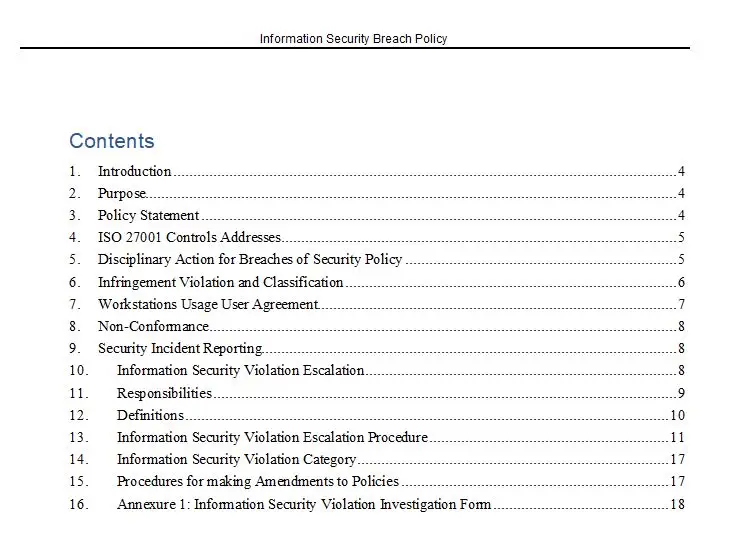
Identification And Traceability Procedures
In the intricate world of medical device manufacturing, where precision and compliance are paramount, the “Identification And Traceability Procedures” stand as a beacon of reliability and assurance. This product, identified by the number 8022, is not just a set of guidelines but a comprehensive framework designed to ensure that every product is meticulously tracked and identified throughout its lifecycle, in strict adherence to ISO 13485 standards.
At the heart of these procedures lies a commitment to quality and safety, essential in the medical device industry. The ISO 13485 standard is globally recognized for its rigorous requirements, and these procedures are crafted to meet and exceed those expectations. They provide a structured approach to product identification and traceability, ensuring that every component, from raw materials to finished products, is accounted for and can be traced back through the supply chain.
Key features of the Identification And Traceability Procedures include a robust system for assigning unique identifiers to each product, enabling seamless tracking and record-keeping. This system not only facilitates compliance with regulatory requirements but also enhances operational efficiency by reducing errors and streamlining processes. The procedures also incorporate advanced technologies for data capture and management, ensuring that information is accurate, up-to-date, and easily accessible.
The benefits of implementing these procedures are manifold. For manufacturers, they offer peace of mind, knowing that their products are compliant with international standards and that they can quickly respond to any issues that may arise. For consumers, they provide an added layer of safety, as products can be traced back to their origin, ensuring accountability and transparency.
The value proposition of the Identification And Traceability Procedures is clear: they are an indispensable tool for any organization seeking to maintain the highest standards of quality and compliance in the medical device industry. By integrating these procedures into their operations, companies can not only meet regulatory requirements but also gain a competitive edge by demonstrating their commitment to excellence and customer safety.
In a world where the stakes are high and the margin for error is slim, the Identification And Traceability Procedures offer a reliable solution for navigating the complexities of product compliance and traceability. They are more than just a set of guidelines; they are a strategic asset that empowers organizations to achieve their goals while safeguarding the well-being of their customers.
All GovernanaceDocs documents are developed based on well-known standards such as NIST CSF, ISO 27001, ISO 22301, PCI-DSS and HIPAA.
Hence, You just need to download and selected document and add your company name and logo.







Reviews
There are no reviews yet